

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116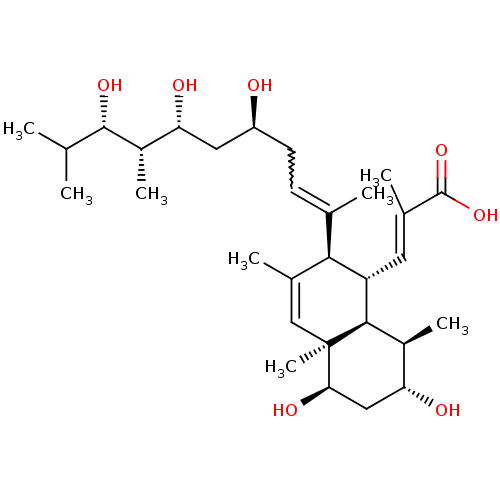 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) | J Med Chem 57: 6822-33 (2014) Article DOI: 10.1021/jm500871s BindingDB Entry DOI: 10.7270/Q2J9681Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) using H4 (1 to 24) as substrate assessed as incorporation of [3H]-methyl group from [3H-Me]-SAM to peptide subst... | J Med Chem 58: 1596-629 (2015) Article DOI: 10.1021/jm501234a BindingDB Entry DOI: 10.7270/Q28K7BS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Punjabi University Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) using biotinylated-H3 peptide as substrate measured after 1 hr in presence of [3H]-SAM by scintillation proximit... | Eur J Med Chem 166: 351-368 (2019) Article DOI: 10.1016/j.ejmech.2019.01.069 BindingDB Entry DOI: 10.7270/Q2PZ5D8X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai Curated by ChEMBL | Assay Description Inhibition of human SETD8 catalytic domain expressed in Escherichia coli assessed as reduction in transfer of tritiated methyl group from [3H]SAM to ... | Medchemcomm 5: 1892-1898 (2014) Article DOI: 10.1039/c4md00317a BindingDB Entry DOI: 10.7270/Q2FR00M9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Center for Combinatorial Chemistry and Drug Discovery of Jilin University Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) using biotin-labeled histone H4 (1 to 24 residues) as substrate incubated for 1 hr in presence of [3H-Me]SAM by ... | Bioorg Med Chem Lett 26: 4436-4440 (2016) Article DOI: 10.1016/j.bmcl.2016.08.004 BindingDB Entry DOI: 10.7270/Q27H1P3Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-lysine methyltransferase KMT5A (Homo sapiens (Human)) | BDBM50051116 (CHEMBL3318284) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of SETD8 (unknown origin) using biotin-labeled H4 (1 to 24 residues) as substrate after 1 hr in presence of [3H]SAM by scintillation proxi... | ACS Med Chem Lett 7: 1102-1106 (2016) Article DOI: 10.1021/acsmedchemlett.6b00303 BindingDB Entry DOI: 10.7270/Q2319XVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||