

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM50063478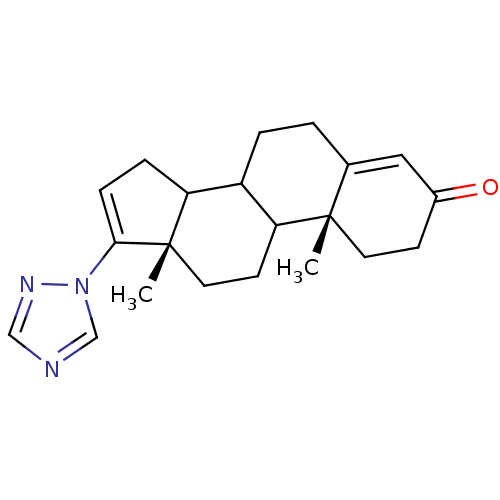 ((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of rat Cytochrome P450 17A1 | J Med Chem 41: 902-12 (1998) Article DOI: 10.1021/jm970568r BindingDB Entry DOI: 10.7270/Q2GH9H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063478 ((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland Curated by ChEMBL | Assay Description Inhibition of human testicular microsomal Cytochrome P450 17A1 | J Med Chem 41: 902-12 (1998) Article DOI: 10.1021/jm970568r BindingDB Entry DOI: 10.7270/Q2GH9H2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM50063478 ((10R,13S)-10,13-Dimethyl-17-[1,2,4]triazol-1-yl-1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Accelrys Curated by ChEMBL | Assay Description In vitro cytochrome P450 17A1 inhibition was assayed using the rapid acetic acid releasing assay (AARA), utilizing intact P450c17-expressing Escheric... | J Med Chem 46: 2345-51 (2003) Article DOI: 10.1021/jm020576u BindingDB Entry DOI: 10.7270/Q2WD41B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||