

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50065965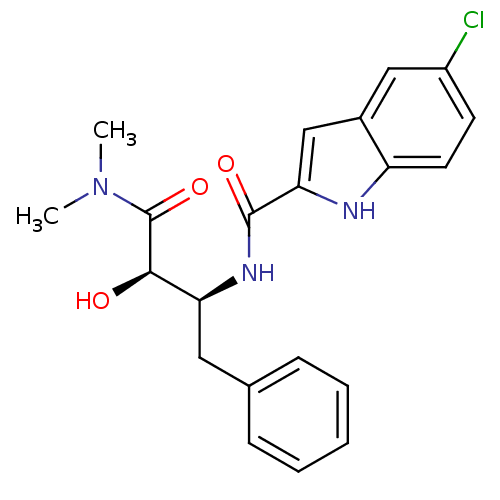 (5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Egypt National Research Centre Curated by ChEMBL | Assay Description Inhibition of human recombinant liver glycogen phosphorylase A expressed in baculovirus infected Sf9 insect cells assessed as release of phosphate fr... | Bioorg Med Chem 24: 5423-5430 (2016) Article DOI: 10.1016/j.bmc.2016.08.069 BindingDB Entry DOI: 10.7270/Q26D5VZD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50065965 (5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description In vitro inhibitory activity against recombinant human liver glycogen phosphorylase a (rHLGPa) catalyzed release of phosphate from glucose-1-phosphat... | J Med Chem 41: 2934-8 (1998) Article DOI: 10.1021/jm980264k BindingDB Entry DOI: 10.7270/Q2NS0T1T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50065965 (5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against glycogen phosphorylase | Bioorg Med Chem Lett 15: 459-65 (2004) Article DOI: 10.1016/j.bmcl.2004.10.048 BindingDB Entry DOI: 10.7270/Q26W99K6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Mus musculus) | BDBM50065965 (5-Chloro-1H-indole-2-carboxylic acid ((1S,2R)-1-be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibitory concentration against glycogen phosphorylase | J Med Chem 47: 3463-82 (2004) Article DOI: 10.1021/jm040031v BindingDB Entry DOI: 10.7270/Q2NC61ZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||