

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycogen phosphorylase, muscle form (Homo sapiens (Human)) | BDBM50136414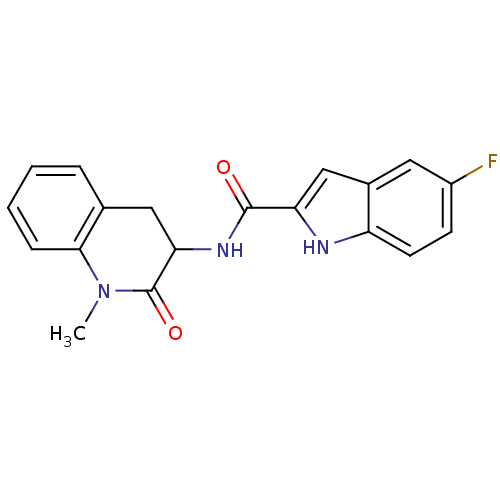 (5-Fluoro-1H-indole-2-carboxylic acid (1-methyl-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human muscle glycogen phosphorylase a (HMGPa) | Bioorg Med Chem Lett 13: 4385-8 (2003) BindingDB Entry DOI: 10.7270/Q2S75FQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50136414 (5-Fluoro-1H-indole-2-carboxylic acid (1-methyl-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Applied Science University Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase 1a assessed as release of phosphate from glucose-1- phosphate after 20 mins | Bioorg Med Chem 19: 4746-71 (2011) Article DOI: 10.1016/j.bmc.2011.06.086 BindingDB Entry DOI: 10.7270/Q2028RXJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50136414 (5-Fluoro-1H-indole-2-carboxylic acid (1-methyl-2-o...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibitory concentration against recombinant human liver glycogen phosphorylase a (HLGPa) | Bioorg Med Chem Lett 13: 4385-8 (2003) BindingDB Entry DOI: 10.7270/Q2S75FQV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||