

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM50160879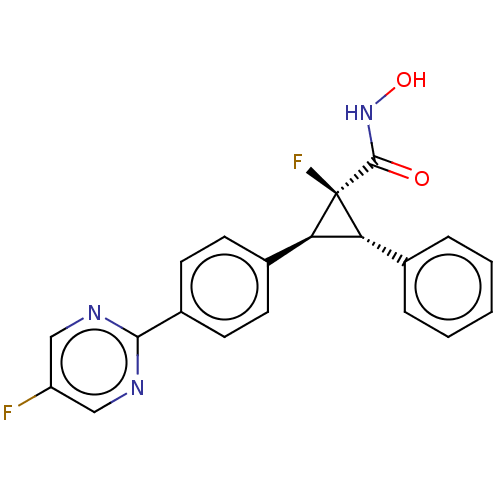 (CHEMBL3793392 | US9505736, (1S,2S,3S)-1-Fluoro-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50160879 (CHEMBL3793392 | US9505736, (1S,2S,3S)-1-Fluoro-2-(...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Phosphodiesterase 2 from pig coronary artery | Citation and Details | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||