

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50166066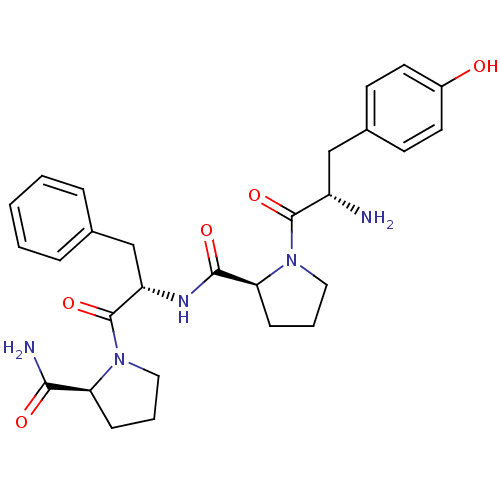 (CHEMBL362991 | H-Tyr-Pro-Phe-Pro-NH2 | MORPHICEPTI...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from Wistar rat mu opioid receptor by liquid scintillation counting | Bioorg Med Chem 19: 6977-81 (2011) Article DOI: 10.1016/j.bmc.2011.10.040 BindingDB Entry DOI: 10.7270/Q2Q52Q1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50166066 (CHEMBL362991 | H-Tyr-Pro-Phe-Pro-NH2 | MORPHICEPTI...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Wilmington Curated by ChEMBL | Assay Description Inhibitory activity against non-selective opiate receptor | Bioorg Med Chem Lett 13: 1029-31 (2003) BindingDB Entry DOI: 10.7270/Q2FB53GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50166066 (CHEMBL362991 | H-Tyr-Pro-Phe-Pro-NH2 | MORPHICEPTI...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 552 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description In vitro inhibitory activity against mu opioid receptor using guinea pig ileum (GPI) assay | J Med Chem 36: 708-19 (1993) BindingDB Entry DOI: 10.7270/Q2Z03761 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50166066 (CHEMBL362991 | H-Tyr-Pro-Phe-Pro-NH2 | MORPHICEPTI...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of Lodz Curated by ChEMBL | Assay Description Displacement of [3H][Ile5,6]deltorphin 2 from delta opioid receptor in Wistar rat brain membrane by liquid scintillation counting | Bioorg Med Chem 19: 6977-81 (2011) Article DOI: 10.1016/j.bmc.2011.10.040 BindingDB Entry DOI: 10.7270/Q2Q52Q1R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50166066 (CHEMBL362991 | H-Tyr-Pro-Phe-Pro-NH2 | MORPHICEPTI...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description In vitro inhibitory activity against delta opioid receptor using mouse vas deferens (MVD) assay | J Med Chem 36: 708-19 (1993) BindingDB Entry DOI: 10.7270/Q2Z03761 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||