

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50190553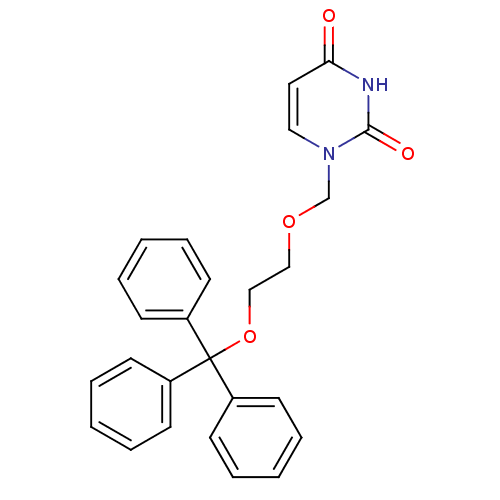 (1-[2-(trityloxy)ethoxymethyl]uracil | CHEMBL424704...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co. Ltd. Curated by ChEMBL | Assay Description Inhibition of human dUTPase assessed as production of [5-3H]dUMP from [5-3H]dUTP after 15 mins measured by HPLC analysis | J Med Chem 55: 2970-80 (2012) Article DOI: 10.1021/jm201628y BindingDB Entry DOI: 10.7270/Q2C82BB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Deoxyuridine 5'-triphosphate nucleotidohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50190553 (1-[2-(trityloxy)ethoxymethyl]uracil | CHEMBL424704...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | US Patent | n/a | n/a | 1.85E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Taiho Pharmaceutical Co., Ltd. US Patent | Assay Description The inhibitory activity of the compound against human dUTPase was determined by measuring the production of [5-3H]deoxyuridine monophosphate from [5-... | US Patent US8530490 (2013) BindingDB Entry DOI: 10.7270/Q25H7DX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||