 Found 4 hits of ic50 for monomerid = 50229697
Found 4 hits of ic50 for monomerid = 50229697 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229697
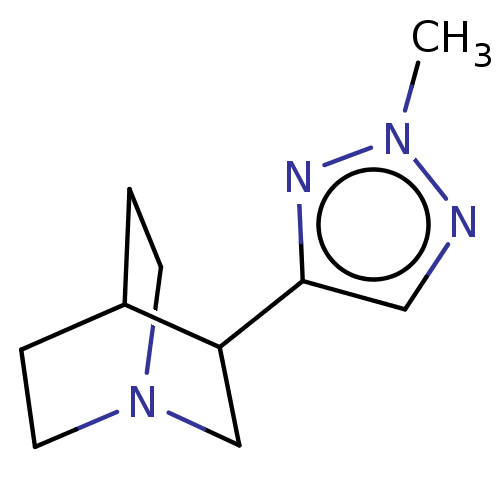
(CHEMBL23721)Show SMILES Cn1ncc(n1)C1CN2CCC1CC2 |(18.33,-12.98,;17.57,-14.14,;18.65,-15.55,;17.46,-16.88,;16.04,-16.24,;16.2,-14.7,;14.7,-17.02,;14.7,-18.55,;13.34,-19.21,;12.03,-18.55,;12.03,-17.02,;13.35,-16.24,;13.75,-17.74,;12.73,-18.07,)| Show InChI InChI=1S/C10H16N4/c1-13-11-6-10(12-13)9-7-14-4-2-8(9)3-5-14/h6,8-9H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]OXO-M binding to Muscarinic receptor from rat cortical homogenates |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229697

(CHEMBL23721)Show SMILES Cn1ncc(n1)C1CN2CCC1CC2 |(18.33,-12.98,;17.57,-14.14,;18.65,-15.55,;17.46,-16.88,;16.04,-16.24,;16.2,-14.7,;14.7,-17.02,;14.7,-18.55,;13.34,-19.21,;12.03,-18.55,;12.03,-17.02,;13.35,-16.24,;13.75,-17.74,;12.73,-18.07,)| Show InChI InChI=1S/C10H16N4/c1-13-11-6-10(12-13)9-7-14-4-2-8(9)3-5-14/h6,8-9H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro ability to displace [3H]oxotremorine-M (OXO-M) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229697

(CHEMBL23721)Show SMILES Cn1ncc(n1)C1CN2CCC1CC2 |(18.33,-12.98,;17.57,-14.14,;18.65,-15.55,;17.46,-16.88,;16.04,-16.24,;16.2,-14.7,;14.7,-17.02,;14.7,-18.55,;13.34,-19.21,;12.03,-18.55,;12.03,-17.02,;13.35,-16.24,;13.75,-17.74,;12.73,-18.07,)| Show InChI InChI=1S/C10H16N4/c1-13-11-6-10(12-13)9-7-14-4-2-8(9)3-5-14/h6,8-9H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]QNB binding to Muscarinic receptor from rat cortical homogenates |
J Med Chem 35: 1280-90 (1992)
BindingDB Entry DOI: 10.7270/Q2G1632Q |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50229697

(CHEMBL23721)Show SMILES Cn1ncc(n1)C1CN2CCC1CC2 |(18.33,-12.98,;17.57,-14.14,;18.65,-15.55,;17.46,-16.88,;16.04,-16.24,;16.2,-14.7,;14.7,-17.02,;14.7,-18.55,;13.34,-19.21,;12.03,-18.55,;12.03,-17.02,;13.35,-16.24,;13.75,-17.74,;12.73,-18.07,)| Show InChI InChI=1S/C10H16N4/c1-13-11-6-10(12-13)9-7-14-4-2-8(9)3-5-14/h6,8-9H,2-5,7H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals
Curated by ChEMBL
| Assay Description
In vitro displacement of [3H]quinuclidinyl benzilate (QNB) from rat cerebral cortex muscarinic receptor. |
J Med Chem 35: 2392-406 (1992)
BindingDB Entry DOI: 10.7270/Q2TF00K5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data