

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50236244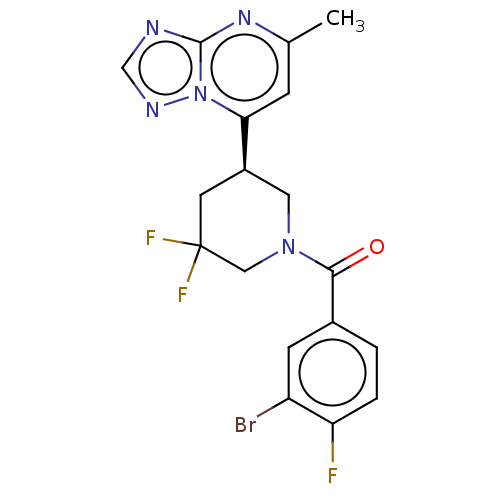 (CHEMBL4065588 | US11186582, Example 268) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Dart Neuroscience LLC Curated by ChEMBL | Assay Description Inhibition of human full length GST-tagged PDE2a using FAM-labeled cAMP as substrate preincubated for 5 mins followed by substrate addition measured ... | J Med Chem 60: 2037-2051 (2017) Article DOI: 10.1021/acs.jmedchem.6b01793 BindingDB Entry DOI: 10.7270/Q2Q242GF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-dependent 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50236244 (CHEMBL4065588 | US11186582, Example 268) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description An IMAP TR-FRET-based phosphodiesterase assay was developed using the PDE2A isoform. IMAP technology is based on high-affinity binding of phosphate b... | Citation and Details BindingDB Entry DOI: 10.7270/Q2988B5K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||