

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transcriptional activator protein LasR (Pseudomonas aeruginosa) | BDBM50241431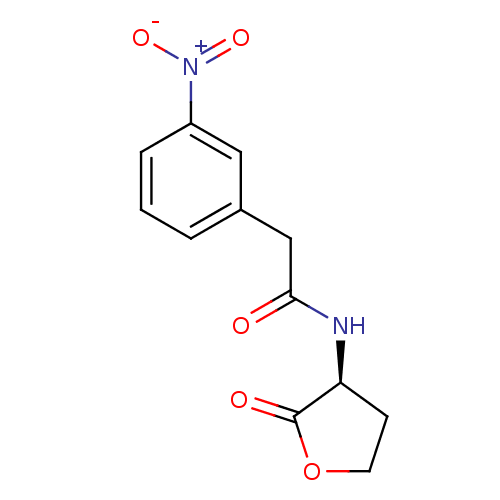 ((S)-2-(3-nitrophenyl)-N-(2-oxo-tetrahydrofuran-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham Curated by ChEMBL | Assay Description Antagonist activity at Pseudomonas aeruginosa LasR expressed in Escherichia coli DH5alpha assessed as inhibition of protein interaction with OdDHL af... | J Med Chem 61: 10385-10402 (2018) Article DOI: 10.1021/acs.jmedchem.8b00540 BindingDB Entry DOI: 10.7270/Q2XP77NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcriptional activator protein LasR (Pseudomonas aeruginosa) | BDBM50241431 ((S)-2-(3-nitrophenyl)-N-(2-oxo-tetrahydrofuran-3-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Florida International University Curated by ChEMBL | Assay Description Inhibition of Pseudomonas aeruginosa LasR | Bioorg Med Chem 19: 5500-6 (2011) Article DOI: 10.1016/j.bmc.2011.07.044 BindingDB Entry DOI: 10.7270/Q2QF8T7Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||