 Found 7 hits of ic50 for monomerid = 50242740
Found 7 hits of ic50 for monomerid = 50242740 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase Fes/Fps
(Homo sapiens (Human)) | BDBM50242740
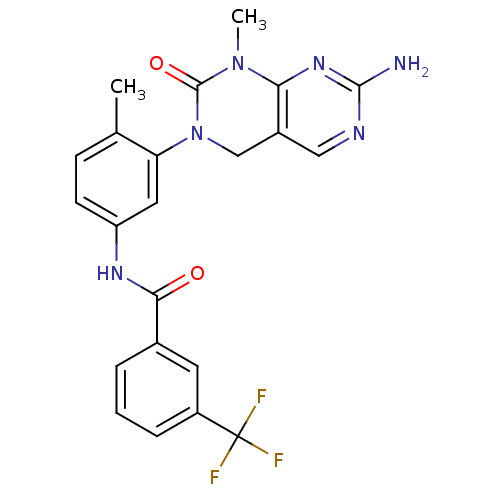
(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Fes |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Cytoplasmic tyrosine-protein kinase BMX
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of Bmx |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1 [1-999,Q252H]
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute
| Assay Description
An in vitro kinase assay using dephophorylated conformationally inactive Abl kinase was obtained by treating pure recombinant Abl with Yersinia ester... |
Chem Biol 13: 779-86 (2006)
Article DOI: 10.1016/j.chembiol.2006.05.015
BindingDB Entry DOI: 10.7270/Q2JM2836 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1 [1-999,Q252H]
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | n/a |
The Scripps Research Institute
| Assay Description
An in vitro kinase assay using phosphorylated recombinant human Abl kinase domains to phosphorylate a peptide substrate. |
Chem Biol 13: 779-86 (2006)
Article DOI: 10.1016/j.chembiol.2006.05.015
BindingDB Entry DOI: 10.7270/Q2JM2836 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 3
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
Curated by ChEMBL
| Assay Description
Inhibition of FGFR3 |
Proc Natl Acad Sci USA 104: 19936-41 (2007)
Article DOI: 10.1073/pnas.0707498104
BindingDB Entry DOI: 10.7270/Q24X58QS |
More data for this
Ligand-Target Pair | |
Activated CDC42 kinase 1
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of ACK1 (unknown origin) by radiometric biochemical kinase assay |
J Med Chem 61: 8353-8373 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00882
BindingDB Entry DOI: 10.7270/Q2T72M31 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase kinase 2
(Homo sapiens (Human)) | BDBM50242740

(CHEMBL459850 | N-(3-(7-Amino-1-methyl-2-oxo-1,2-di...)Show SMILES CN1C(=O)N(Cc2cnc(N)nc12)c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1C Show InChI InChI=1S/C22H19F3N6O2/c1-12-6-7-16(28-19(32)13-4-3-5-15(8-13)22(23,24)25)9-17(12)31-11-14-10-27-20(26)29-18(14)30(2)21(31)33/h3-10H,11H2,1-2H3,(H,28,32)(H2,26,27,29) | MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea University
Curated by ChEMBL
| Assay Description
Inhibition of GCK (unknown origin) by radiometric biochemical kinase assay |
J Med Chem 61: 8353-8373 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00882
BindingDB Entry DOI: 10.7270/Q2T72M31 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data