

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leucine-rich repeat serine/threonine-protein kinase 2 (Homo sapiens (Human)) | BDBM50337134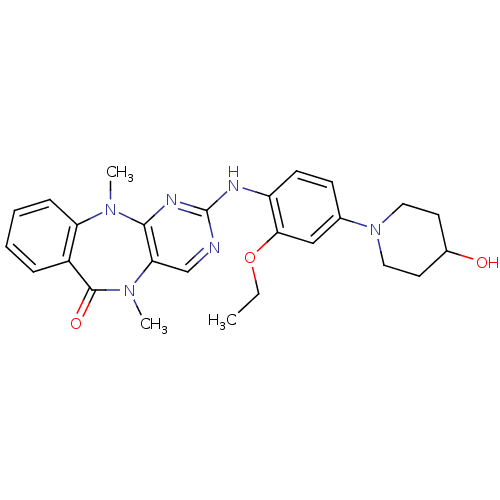 (2-((2-ethoxy-4-(4-hydroxypiperidin-1-yl)phenyl)ami...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human GST-tagged LRRK2 catalytic domain (970 to 2527 residues) expressed in baculovirus expression system using LRRKtide as... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 7 (Homo sapiens (Human)) | BDBM50337134 (2-((2-ethoxy-4-(4-hydroxypiperidin-1-yl)phenyl)ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of His-tagged MAP2K5 activated N-terminal GST-tagged recombinant human ERK5 (1 to 398 residues) expressed in Escherichia coli using biotin... | J Med Chem 62: 928-940 (2019) Article DOI: 10.1021/acs.jmedchem.8b01606 BindingDB Entry DOI: 10.7270/Q2FN19H1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 7 (Homo sapiens (Human)) | BDBM50337134 (2-((2-ethoxy-4-(4-hydroxypiperidin-1-yl)phenyl)ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of EGF-induced BMK1 autophosphorylation in human HeLa cells by SDS-PAGE analysis | ACS Med Chem Lett 2: 195-200 (2011) Article DOI: 10.1021/ml100304b BindingDB Entry DOI: 10.7270/Q2222VSB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 7 (Homo sapiens (Human)) | BDBM50337134 (2-((2-ethoxy-4-(4-hydroxypiperidin-1-yl)phenyl)ami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Newcastle University Curated by ChEMBL | Assay Description Inhibition of ERK5 in human HeLa cells incubated for 15 mins prior to ATP addition by KiNativ profiling method | Eur J Med Chem 178: 530-543 (2019) Article DOI: 10.1016/j.ejmech.2019.05.057 BindingDB Entry DOI: 10.7270/Q23F4T0G | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase DCLK1 (Homo sapiens (Human)) | BDBM50337134 (2-((2-ethoxy-4-(4-hydroxypiperidin-1-yl)phenyl)ami...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 716 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-tagged DCLK1 (G351 to H689 residues) expressed in Escherichia coli BL21 DE3 using 5-FAM-KKLRRTLSVA-CO... | J Med Chem 63: 7817-7826 (2020) Article DOI: 10.1021/acs.jmedchem.0c00596 BindingDB Entry DOI: 10.7270/Q2SB4981 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||