

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50350465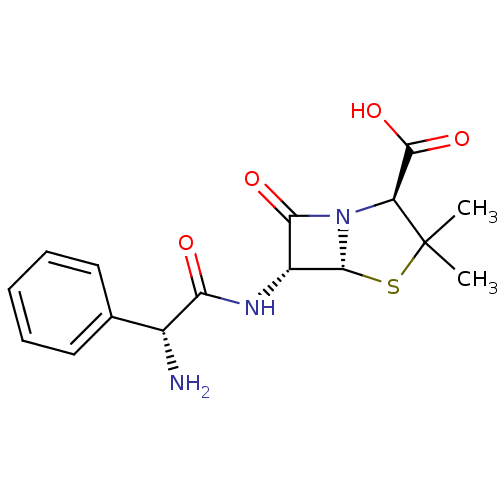 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 28.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lucknow University Curated by ChEMBL | Assay Description Antibacterial activity against Streptococcus faecalis by broth microdilution technique | Bioorg Med Chem Lett 16: 5883-7 (2006) Article DOI: 10.1016/j.bmcl.2006.08.060 BindingDB Entry DOI: 10.7270/Q2MK6GNN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jnatprod.2c00444 BindingDB Entry DOI: 10.7270/Q2H9997N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 8.30E+4 | -23.3 | 5.68E+4 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Erzincan University | Assay Description CA activity was assayed according to method of Verpoorte et al. [Verpoorte et al., J. Biol. Chem., 242:4221-4229] described previously by Innocenti e... | J Enzyme Inhib Med Chem 27: 641-5 (2012) Article DOI: 10.3109/14756366.2011.604852 BindingDB Entry DOI: 10.7270/Q2WH2NXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 60 kDa heat shock protein, mitochondrial (Homo sapiens) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human N-terminal octa-His-tagged HSP60 expressed in Escherichia coli Rosetta(DE3) pLysS/human HSP10 expressed in Escherichia coli Roset... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Co-chaperonin GroES (Escherichia coli) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coli DH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed ... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Co-chaperonin GroES (Escherichia coli) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of Escherichia coli GroEL expressed in Escherichia coli DH5alpha/Escherichia coli GroES expressed in Escherichia coli BL21 (DE3) assessed ... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thiosulfate sulfurtransferase (Homo sapiens) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Indiana University School of Medicine Curated by ChEMBL | Assay Description Inhibition of refolded rhodanese (unknown origin) preincubated with Escherichia coli GroEL/GroES for 60 mins in absence of compound followed by compo... | J Med Chem 61: 10651-10664 (2018) Article DOI: 10.1021/acs.jmedchem.8b01293 BindingDB Entry DOI: 10.7270/Q2MG7S6W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 15 member 1 (Homo sapiens (Human)) | BDBM50350465 (AMPICILLIN | AY-6108 | Amcill | Aminobenzylpenicil...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.00E+7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California San Francisco Curated by ChEMBL | Assay Description TP_TRANSPORTER: inhibition of Gly-Sar uptake (Gly-Sar: 25000 uM) in PEPT1-expressing CHO cells | Pharm Res 13: 1631-4 (1996) Article DOI: 10.1023/a:1016476220296 BindingDB Entry DOI: 10.7270/Q2TM7DZF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||