

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM11640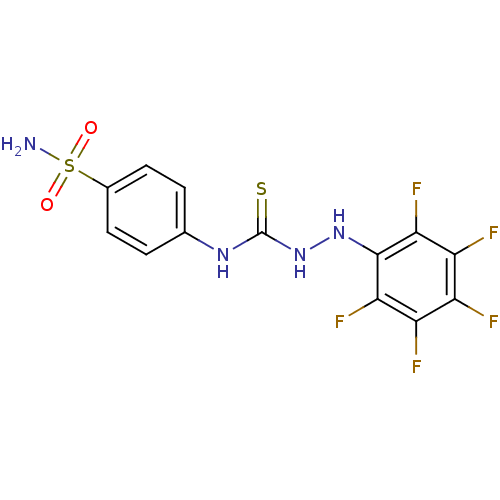 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 5721-7 (2005) Article DOI: 10.1021/jm050333c BindingDB Entry DOI: 10.7270/Q2ST7N2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples 'Federico II' Curated by ChEMBL | Assay Description Inhibitory constant against human Carbonic anhydrase IX | Bioorg Med Chem Lett 15: 1937-42 (2005) Article DOI: 10.1016/j.bmcl.2005.01.086 BindingDB Entry DOI: 10.7270/Q24749CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against catalytic domain of human carbonic anhydrase XII | Bioorg Med Chem Lett 15: 3821-7 (2005) Article DOI: 10.1016/j.bmcl.2005.06.054 BindingDB Entry DOI: 10.7270/Q2H132SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Istituto di Biostrutture e Bioimmagini-CNR | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | J Med Chem 48: 5721-7 (2005) Article DOI: 10.1021/jm050333c BindingDB Entry DOI: 10.7270/Q2ST7N2F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples 'Federico II' Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase II | Bioorg Med Chem Lett 15: 1937-42 (2005) Article DOI: 10.1016/j.bmcl.2005.01.086 BindingDB Entry DOI: 10.7270/Q24749CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase II | Bioorg Med Chem Lett 15: 3821-7 (2005) Article DOI: 10.1016/j.bmcl.2005.06.054 BindingDB Entry DOI: 10.7270/Q2H132SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples 'Federico II' Curated by ChEMBL | Assay Description Inhibitory constant against human Carbonic anhydrase I | Bioorg Med Chem Lett 15: 1937-42 (2005) Article DOI: 10.1016/j.bmcl.2005.01.086 BindingDB Entry DOI: 10.7270/Q24749CF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM11640 (3-[(2,3,4,5,6-pentafluorophenyl)amino]-1-(4-sulfam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi Curated by ChEMBL | Assay Description Inhibitory activity against human carbonic anhydrase I | Bioorg Med Chem Lett 15: 3821-7 (2005) Article DOI: 10.1016/j.bmcl.2005.06.054 BindingDB Entry DOI: 10.7270/Q2H132SW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||