

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor VII (Homo sapiens (Human)) | BDBM13556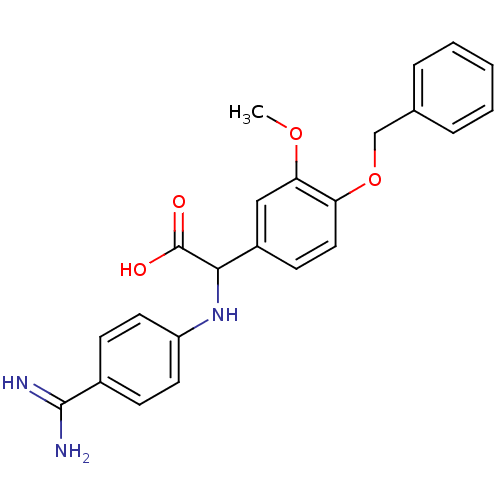 (2-[4-(benzyloxy)-3-methoxyphenyl]-2-[(4-carbamimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 61 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
F. Hoffmann-La Roche Ltd. | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 15: 5344-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.079 BindingDB Entry DOI: 10.7270/Q2X63K6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM13556 (2-[4-(benzyloxy)-3-methoxyphenyl]-2-[(4-carbamimid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 15: 5344-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.079 BindingDB Entry DOI: 10.7270/Q2X63K6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Bos taurus (bovine)) | BDBM13556 (2-[4-(benzyloxy)-3-methoxyphenyl]-2-[(4-carbamimid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 15: 5344-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.079 BindingDB Entry DOI: 10.7270/Q2X63K6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM13556 (2-[4-(benzyloxy)-3-methoxyphenyl]-2-[(4-carbamimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd. | Assay Description The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... | Bioorg Med Chem Lett 15: 5344-52 (2005) Article DOI: 10.1016/j.bmcl.2005.04.079 BindingDB Entry DOI: 10.7270/Q2X63K6X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||