

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM168079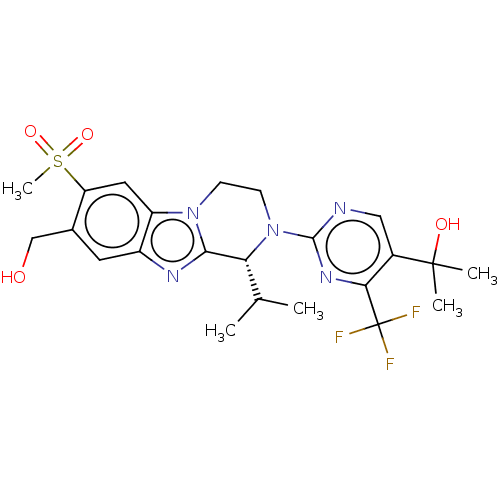 (US9073931, E27a | US9073931, E7a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US9073931 (2015) BindingDB Entry DOI: 10.7270/Q26W98VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta (Homo sapiens (Human)) | BDBM168079 (US9073931, E27a | US9073931, E7a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 14 | -44.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US9073931 (2015) BindingDB Entry DOI: 10.7270/Q26W98VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM168079 (US9073931, E27a | US9073931, E7a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 23 | -43.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US9073931 (2015) BindingDB Entry DOI: 10.7270/Q26W98VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha (Homo sapiens (Human)) | BDBM168079 (US9073931, E27a | US9073931, E7a) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 253 | -37.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Vitae Pharmaceuticals, Inc. US Patent | Assay Description Compounds of the invention were assessed in a competition binding assay where different concentrations of compounds were incubated with the LXR ligan... | US Patent US9073931 (2015) BindingDB Entry DOI: 10.7270/Q26W98VC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||