

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM210749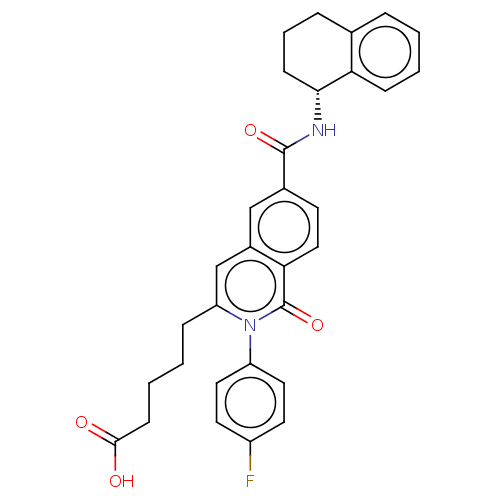 (US9290454, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Antagonist activity at CRTh2 (unknown origin) assessed as inhibition of CD11b activation | Bioorg Med Chem Lett 27: 5344-5348 (2017) Article DOI: 10.1016/j.bmcl.2017.07.064 BindingDB Entry DOI: 10.7270/Q2HX1G7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM210749 (US9290454, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 5.79 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Merck Sharp & Dohme Corp. US Patent | Assay Description Radioligand binding was performed using filtration or scintillation proximity assay (SPA) technology. SPA assays were done at room temperature in 10 ... | US Patent US9290454 (2016) BindingDB Entry DOI: 10.7270/Q2W66JMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM210749 (US9290454, 3.1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Antagonist activity at human CRTh2 expressed in HEK cells assessed as inhibition of DK-PGD2-mediated attenuation of forskolin-induced cAMP accumulati... | Bioorg Med Chem Lett 27: 5344-5348 (2017) Article DOI: 10.1016/j.bmcl.2017.07.064 BindingDB Entry DOI: 10.7270/Q2HX1G7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||