

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM26658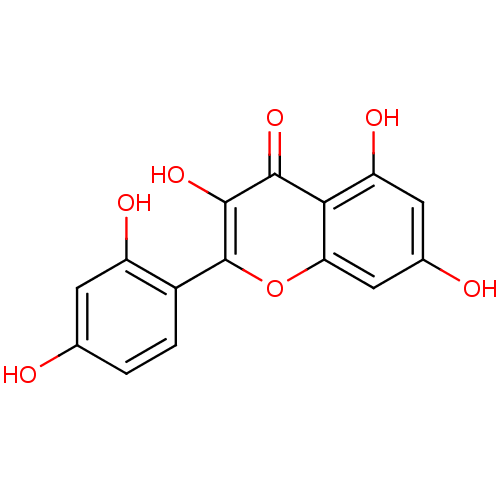 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Solute carrier family 22 member 12 (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Chinese University of Hong Kong Curated by ChEMBL | Assay Description Inhibition of human URAT1-mediated urate uptake in HEK293 cells by competitive inhibition assay | Drug Metab Dispos 35: 981-6 (2007) Article DOI: 10.1124/dmd.106.012187 BindingDB Entry DOI: 10.7270/Q2KK9CPP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Yes (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
West China Hospital of Sichuan University Curated by ChEMBL | Assay Description Inhibition of YES (unknown origin) | Eur J Med Chem 166: 186-196 (2019) Article DOI: 10.1016/j.ejmech.2019.01.043 BindingDB Entry DOI: 10.7270/Q20C50BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Rattus norvegicus (rat)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity at Adenosine A1 receptor in rat brain membranes by [3H]-PIA displacement. | J Med Chem 39: 2293-301 (1996) Article DOI: 10.1021/jm950923i BindingDB Entry DOI: 10.7270/Q2D799J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 3 (Bos taurus (Cattle)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lactoylglutathione lyase (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uniroyal Chemical Co., Inc. Curated by ChEMBL | Assay Description Inhibition constant of compound against binding of Yeast Glyoxalase I | J Med Chem 31: 1396-406 (1988) BindingDB Entry DOI: 10.7270/Q2P55QQJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM26658 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes Curated by ChEMBL | Assay Description Binding affinity against human adenosine A3 receptor in HEK293 cells using [125I]-AB-MECA 21680 radioligand. | J Med Chem 39: 2293-301 (1996) Article DOI: 10.1021/jm950923i BindingDB Entry DOI: 10.7270/Q2D799J2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||