

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50101817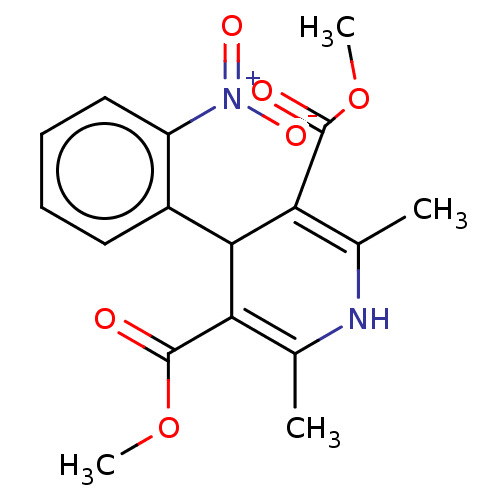 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]nitrendipine binding to L-type calcium channel from rat brain cortex homogenate | J Med Chem 29: 2504-11 (1986) BindingDB Entry DOI: 10.7270/Q2QF8W3R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50101817 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Assay Description Alpha-1 adrenergic receptor binding affinity was evaluated by its ability to displace [3H]prazosin in rat brain synaptosomes | Citation and Details BindingDB Entry DOI: 10.7270/Q22N54GM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50101817 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Equus caballus (horse) serum butyrylcholinesterase (BChE) assessed as inhibition of BTCh hydrolysis by Ellman method | Citation and Details Article DOI: 10.1007/s00044-005-0140-0 BindingDB Entry DOI: 10.7270/Q2CJ8HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50101817 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Equus caballus (horse) serum butyrylcholinesterase (BChE) assessed as inhibition of BTCh hydrolysis by Ellman method | Citation and Details Article DOI: 10.1007/s00044-005-0140-0 BindingDB Entry DOI: 10.7270/Q2CJ8HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50101817 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus (electric eel) acetylcholinesterase (AChE) assessed as inhibition of ATCh hydrolysis by Ellman method | Citation and Details Article DOI: 10.1007/s00044-005-0140-0 BindingDB Entry DOI: 10.7270/Q2CJ8HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50101817 (Adalat | Adalat Cc | Afeditab Cr | BAY-A-1040 | CH...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Electrophorus electricus (electric eel) acetylcholinesterase (AChE) assessed as inhibition of ATCh hydrolysis by Ellman method | Citation and Details Article DOI: 10.1007/s00044-005-0140-0 BindingDB Entry DOI: 10.7270/Q2CJ8HD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||