

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303322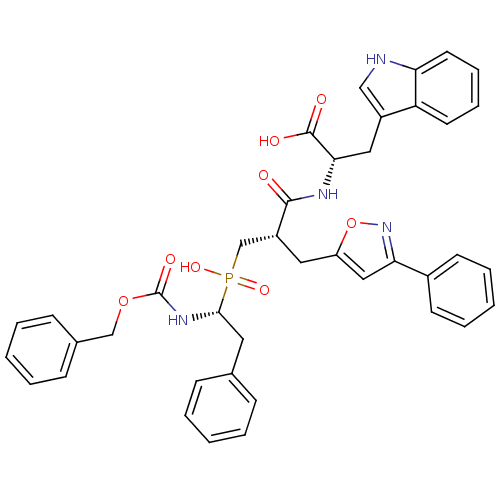 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE C-terminal domain | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ACE C-terminal domain | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human MMP13 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-converting enzyme 1 (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic ECE1 | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic NEP | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50303322 ((2S)-2-({3-[Hydroxyl(2-phenyl-(1R)-1-{[(benzyloxy)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CEA, DSV, Service d'Ingenierie Moleculaire des Proteines (SIMOPRO) Curated by ChEMBL | Assay Description Inhibition of human somatic NEP | J Med Chem 53: 208-20 (2010) Article DOI: 10.1021/jm9010803 BindingDB Entry DOI: 10.7270/Q2736R06 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||