

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proto-oncogene tyrosine-protein kinase receptor Ret (Homo sapiens (Human)) | BDBM50331094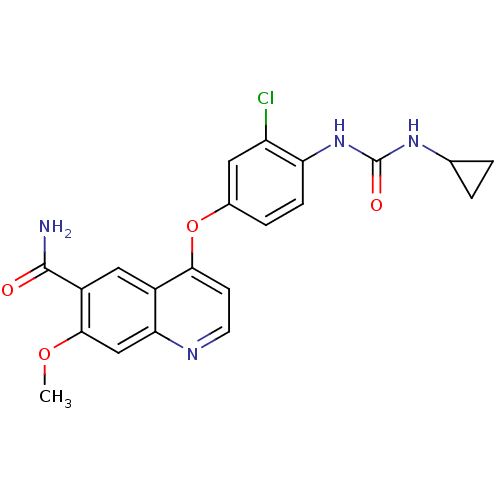 (4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Daegu-Gyeongbuk Medical Innovation Foundation (DGMIF) Curated by ChEMBL | Assay Description Inhibition of RET (unknown origin) | J Med Chem 58: 3672-81 (2015) Article DOI: 10.1021/jm501464c BindingDB Entry DOI: 10.7270/Q2RR2106 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 5A, mitochondrial (Homo sapiens (Human)) | BDBM50331094 (4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | 133 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
"Magna Gr£cia" University of Catanzaro Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase VA preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111565 BindingDB Entry DOI: 10.7270/Q2QF8X7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50331094 (4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
"Magna Gr£cia" University of Catanzaro Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111565 BindingDB Entry DOI: 10.7270/Q2QF8X7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50331094 (4-(3-chloro-4-(3-cyclopropylureido)phenoxy)-7-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
"Magna Gr£cia" University of Catanzaro Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 1 preincubated with enzyme for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111565 BindingDB Entry DOI: 10.7270/Q2QF8X7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||