

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443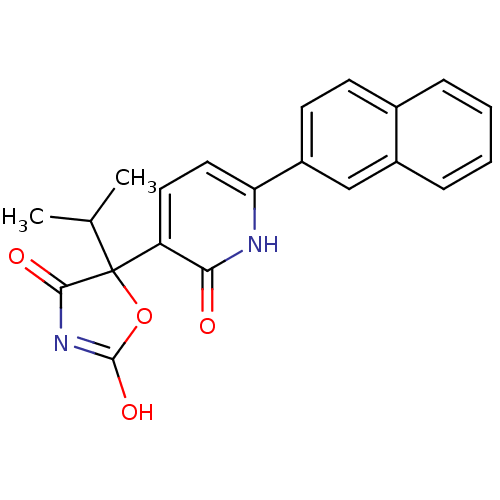 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human EP3 | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-PGE2 from human EP3 expressed in Chem-1 cell membranes incubated for 2 hrs by TopCount scintillation plate reader analysis | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00667 BindingDB Entry DOI: 10.7270/Q2M61PWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (RAT) | BDBM50384443 (CHEMBL1770317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at EP1 receptor in human U2OS cells expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs by FLIPR assay | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||