

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100706 (US8507676, 55) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100690 (US8507676, 37B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439101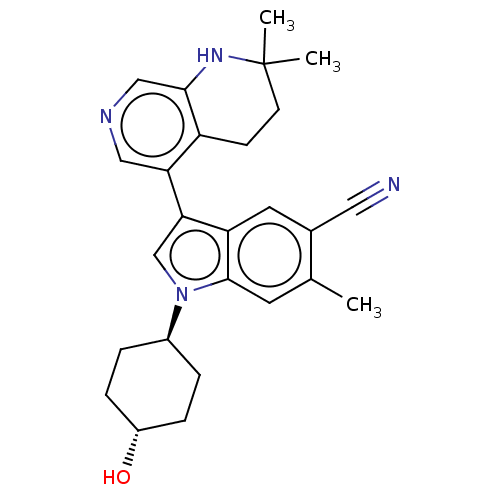 (3-(2,2-dimethyl-1,2,3,4-tetrahydro-1,7-naphthyridi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99246 (US8497368, 38) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99250 (US8497368, 42) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99253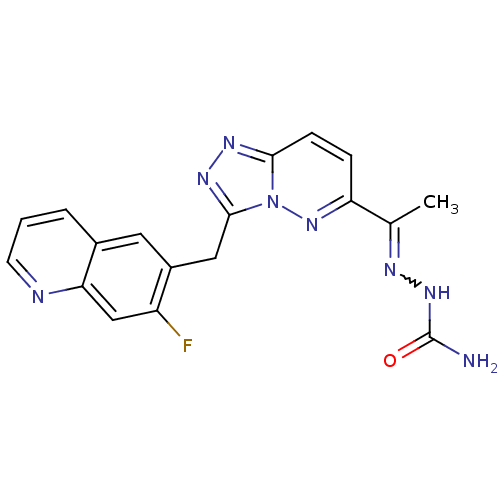 (US8497368, 45) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99263 (US8497368, 55) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99288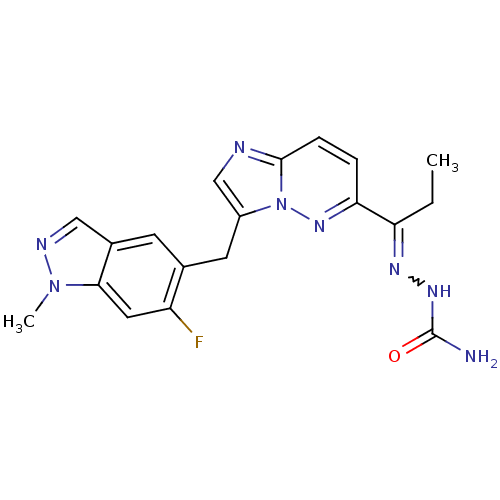 (US8497368, 80) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM302354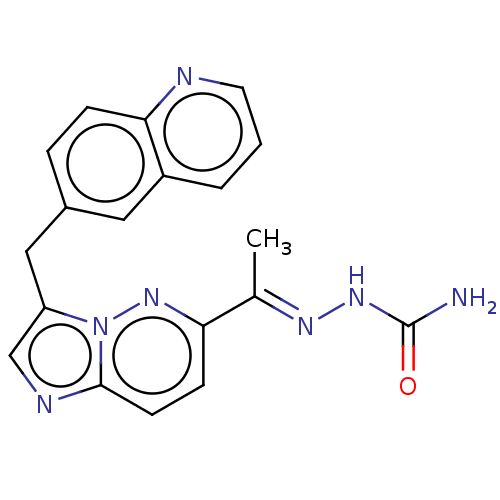 (US8497368, 81) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439046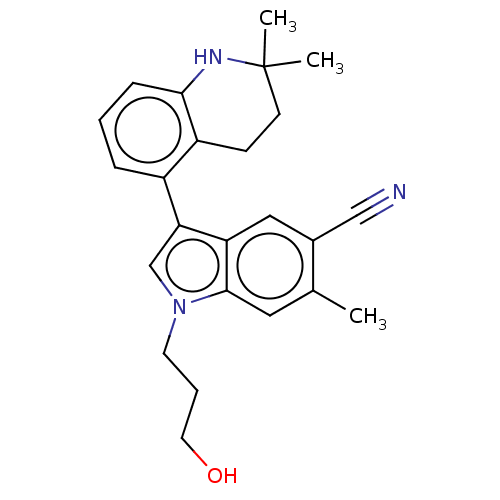 (US10604502, Ex # 34) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439056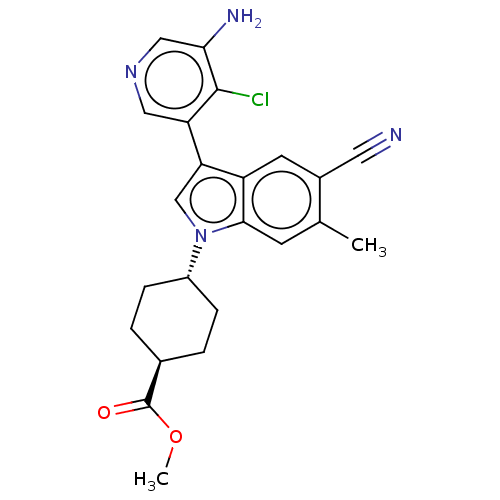 (US10604502, Ex # 44) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100705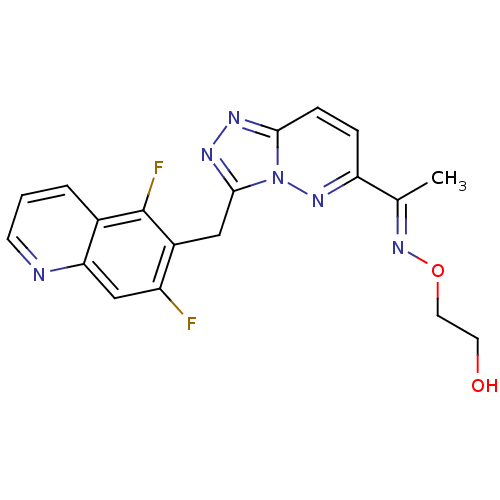 (US8507676, 54 | US8507676, 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99267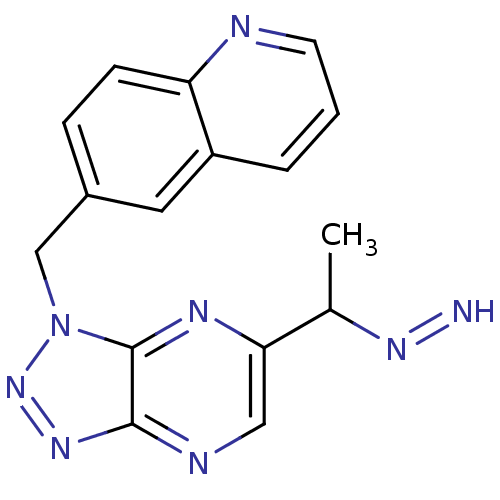 (US8497368, 59) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM99277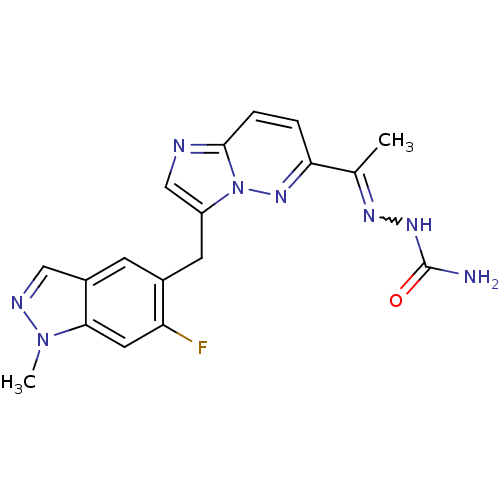 (US8497368, 69) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8497368 (2013) BindingDB Entry DOI: 10.7270/Q2KW5DN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439015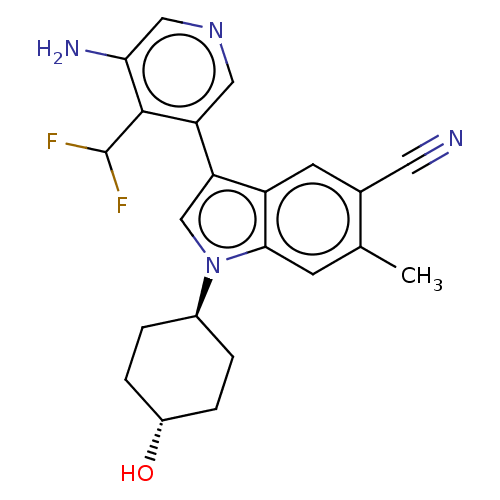 (US10604502, Ex # 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439036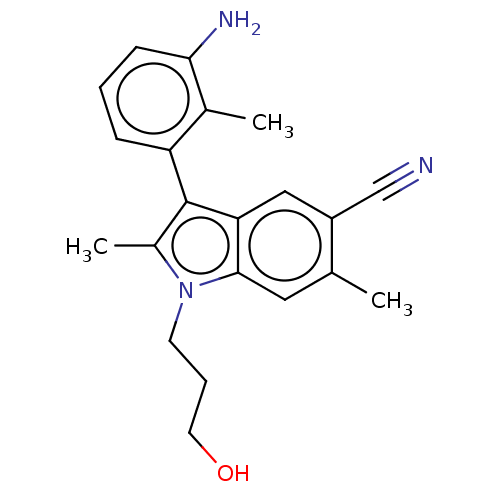 (3-(3-amino-2-methylphenyl)-1-(3-hydroxypropyl)-2,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439121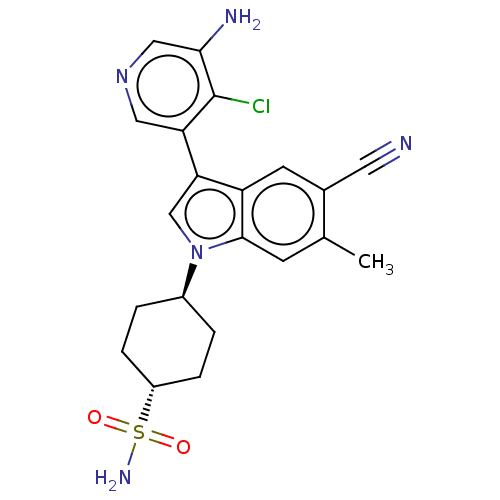 (1-((1R,4R)-1-aminosulfonylcyclohexyl)-6-methyl-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439090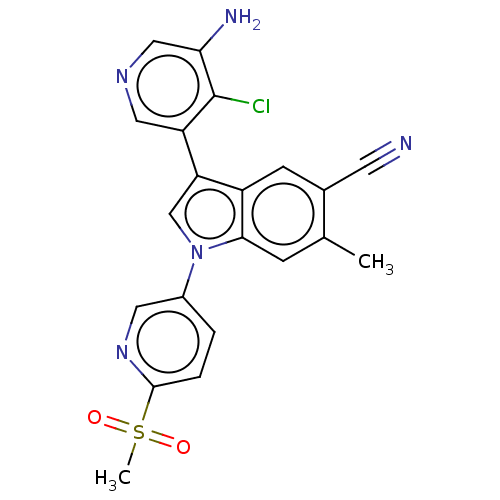 (US10604502, Ex # 78) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM439107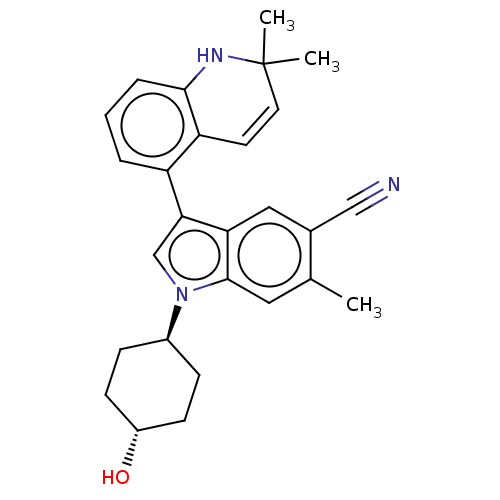 (US10604502, Ex # 95) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Representative compounds of the present invention were serially and separately diluted 3-fold in DMSO to obtain a total of twelve concentrations. The... | US Patent US10604502 (2020) BindingDB Entry DOI: 10.7270/Q2MW2M62 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100672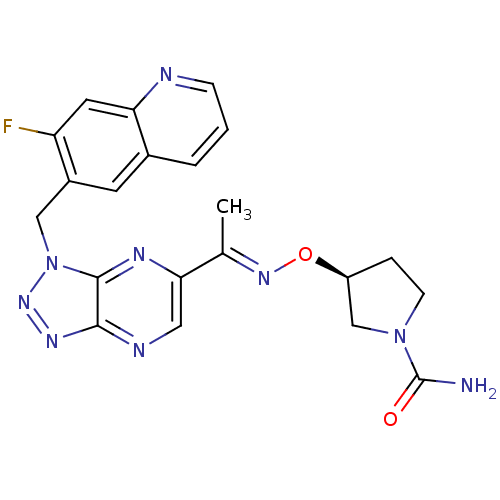 (US8507676, 21) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hepatocyte growth factor receptor (Homo sapiens (Human)) | BDBM100670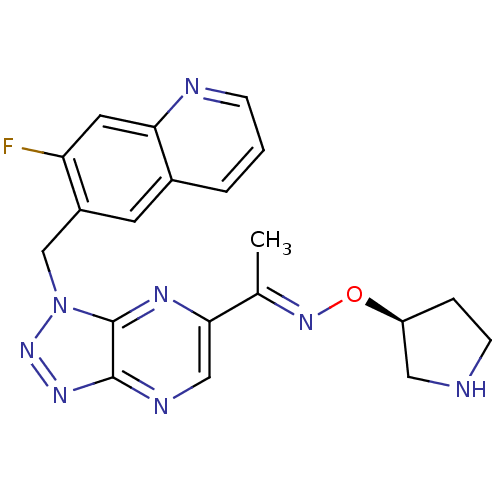 (US8507676, 19) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The kinase assay is based on the LanthaScreen technology. LanthaScreen is the detection of Time-Resolved Fluorescence Resonance Energy Transfer (TR-... | US Patent US8507676 (2013) BindingDB Entry DOI: 10.7270/Q2MS3RC9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568073 (US11420970, Example 275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568076 (US11420970, Example 278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568077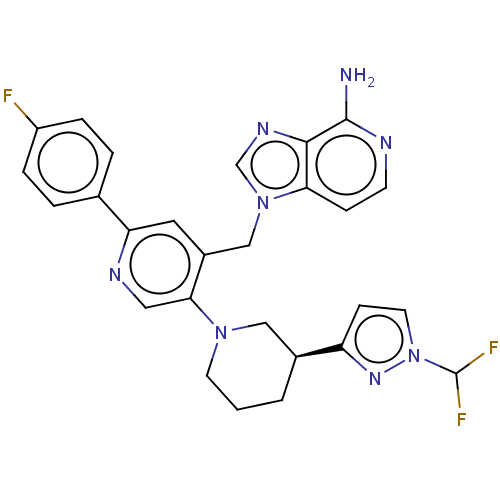 (US11420970, Example 279) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568078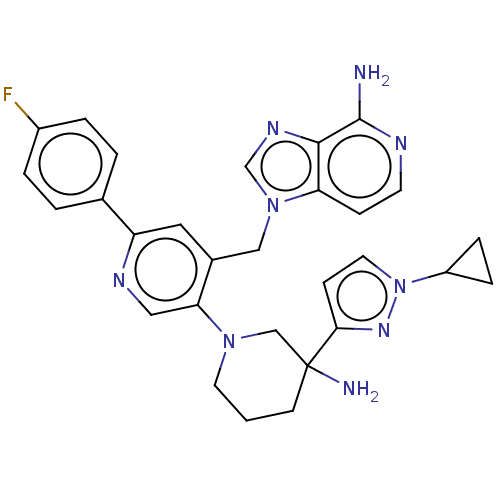 (US11420970, Example 280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568081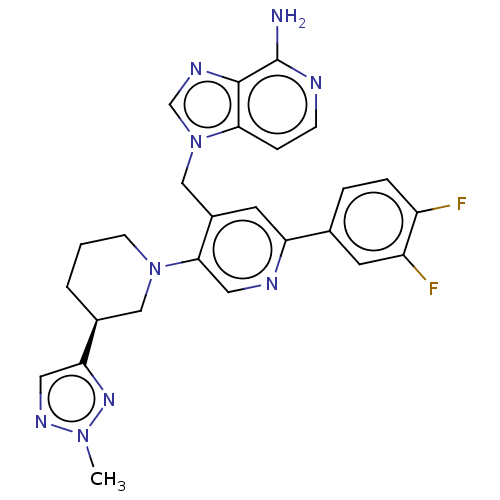 (US11420970, Example 283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568082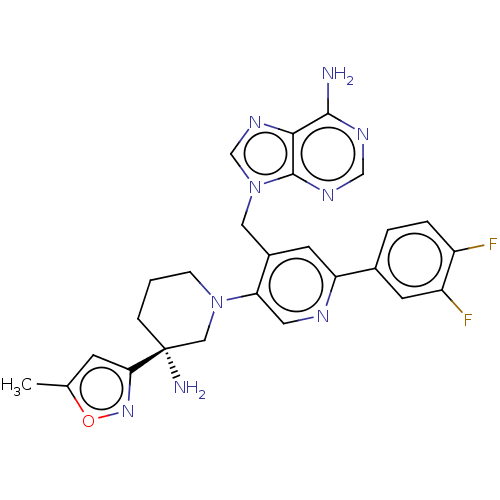 (US11420970, Example 284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568083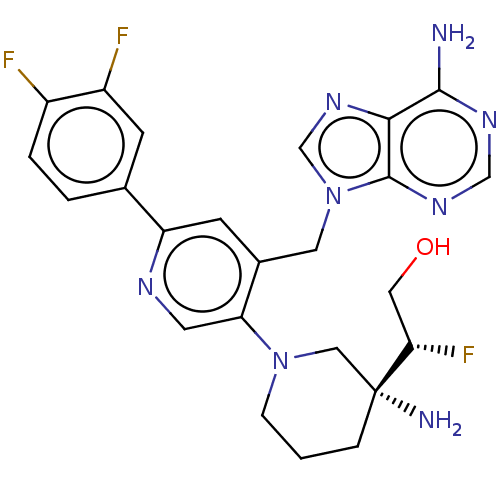 (US11420970, Example 285 | US11420970, Example 289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568084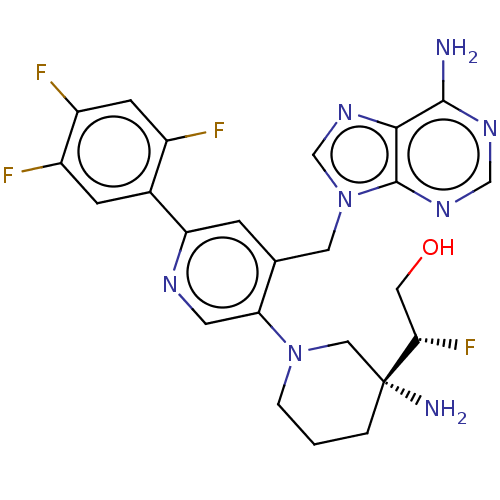 (US11420970, Example 286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM568083 (US11420970, Example 285 | US11420970, Example 289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567821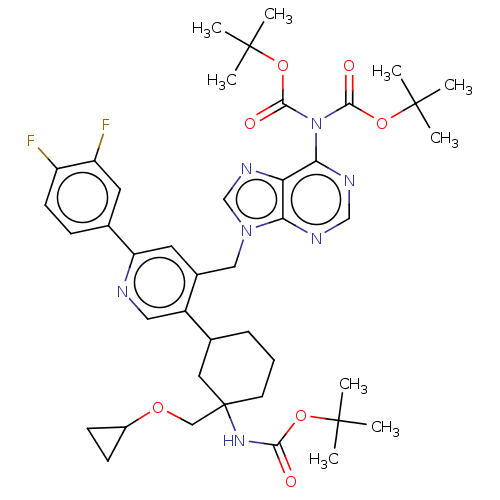 (US11420970, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567822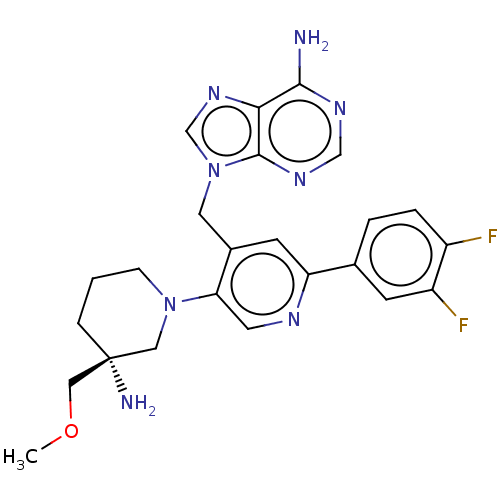 (US11420970, Example 23) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567823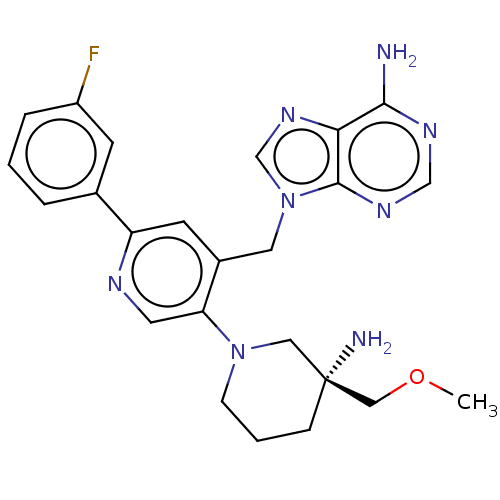 (US11420970, Example 24) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567824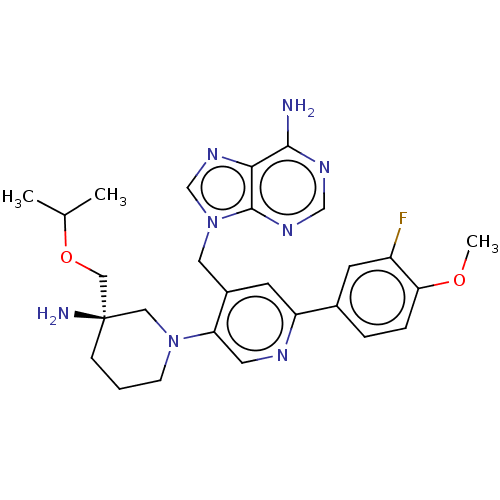 (US11420970, Example 25) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567825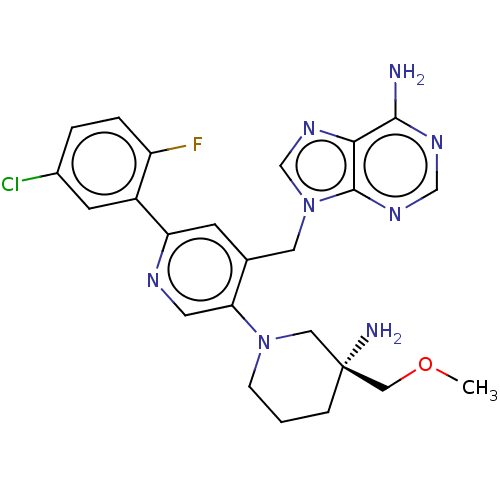 (US11420970, Example 26) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567826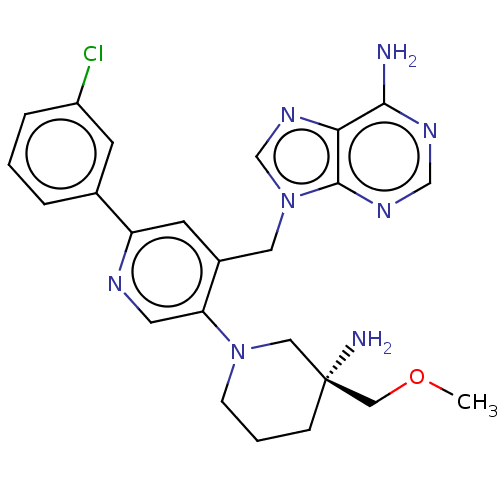 (US11420970, Example 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567827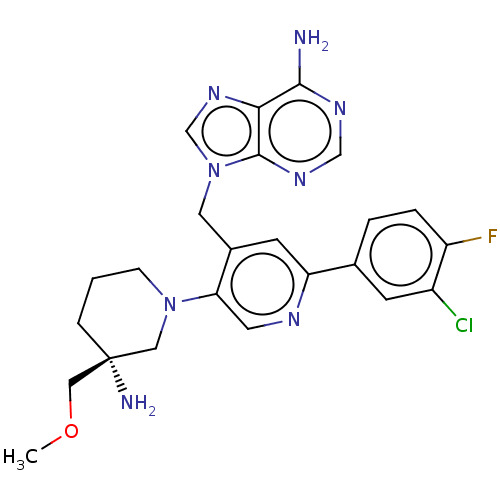 (US11420970, Example 28) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567828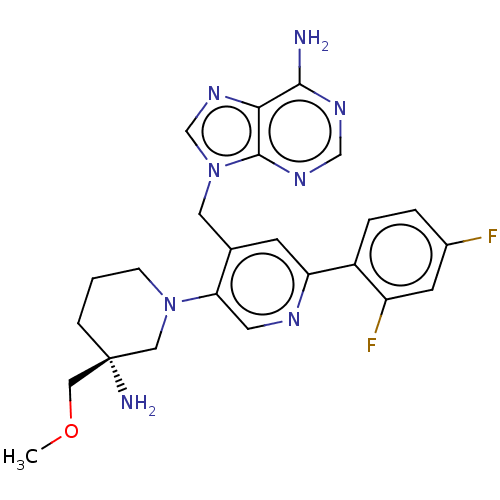 (US11420970, Example 29) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567829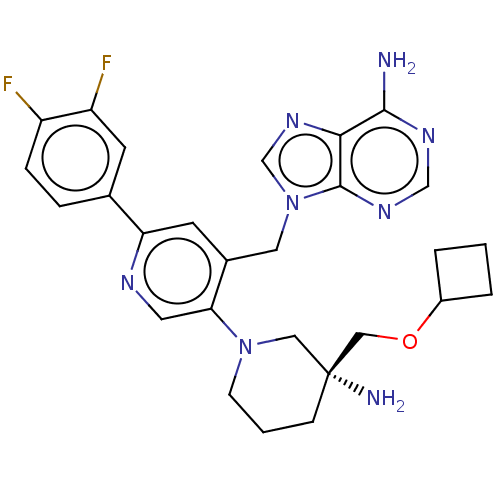 (US11420970, Example 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567830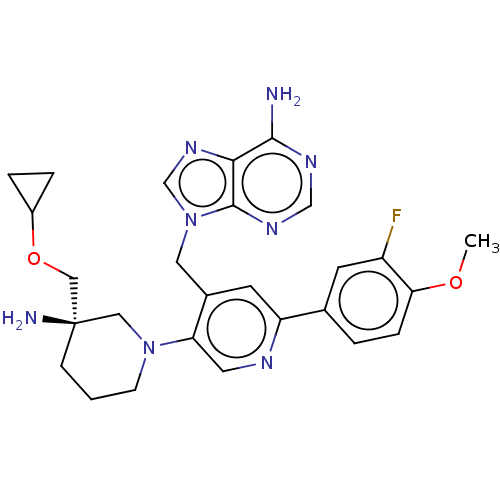 (US11420970, Example 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567831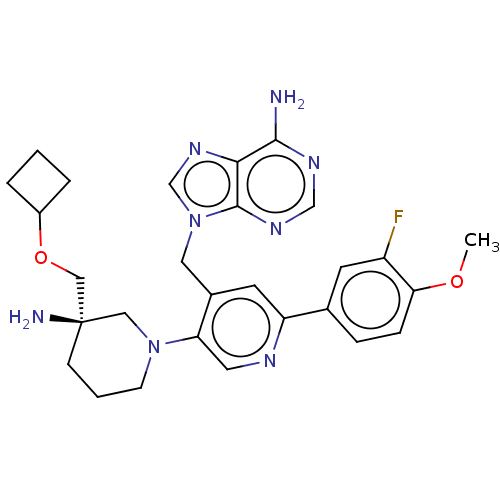 (US11420970, Example 32) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567832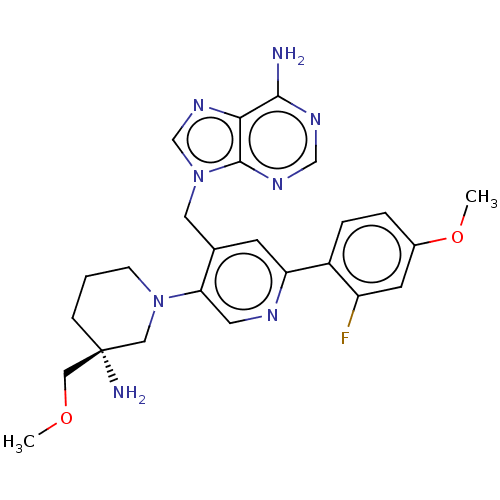 (US11420970, Example 33) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567833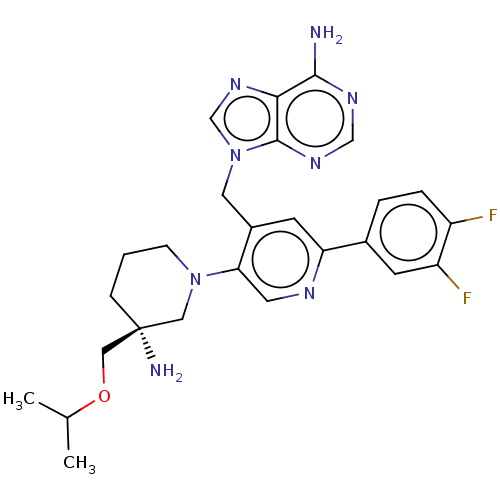 (US11420970, Example 34) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567834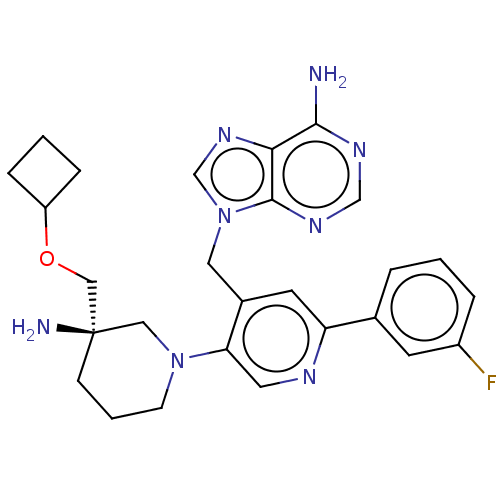 (US11420970, Example 35) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567837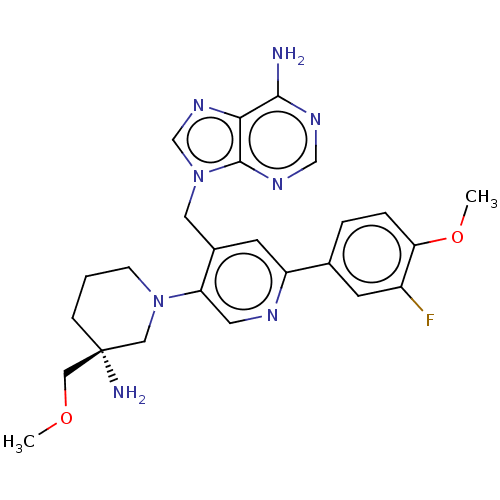 (US11420970, Example 38 | US11420970, Example 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567847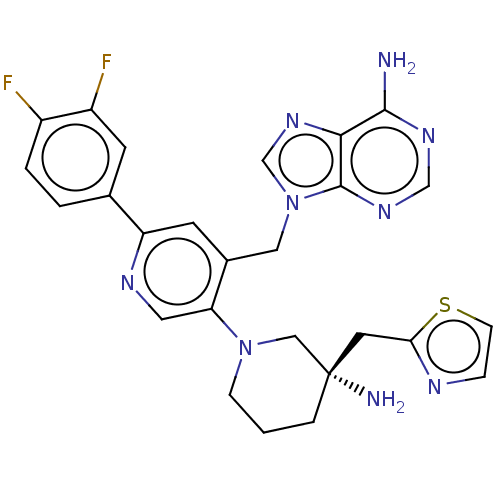 (US11420970, Example 48 | US11420970, Example 53) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567848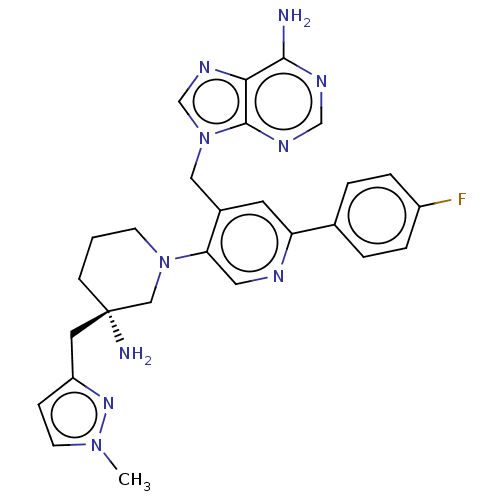 (US11420970, Example 49) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567849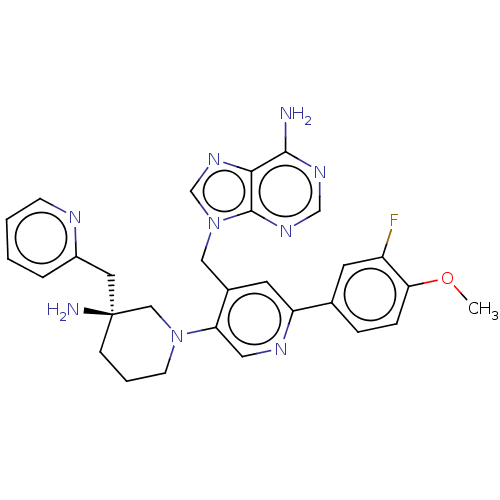 (US11420970, Example 50) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567850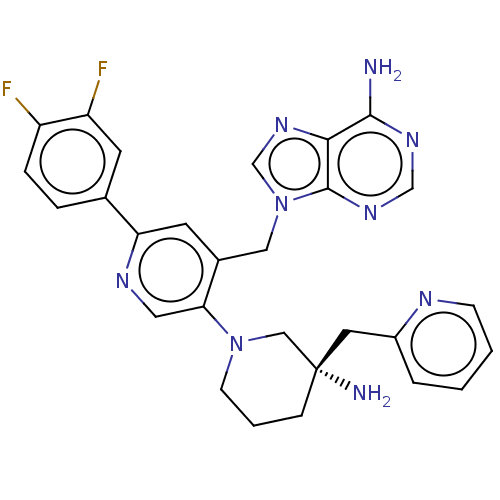 (US11420970, Example 51 | US11420970, Example 54) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase NSD2 (Homo sapiens (Human)) | BDBM567851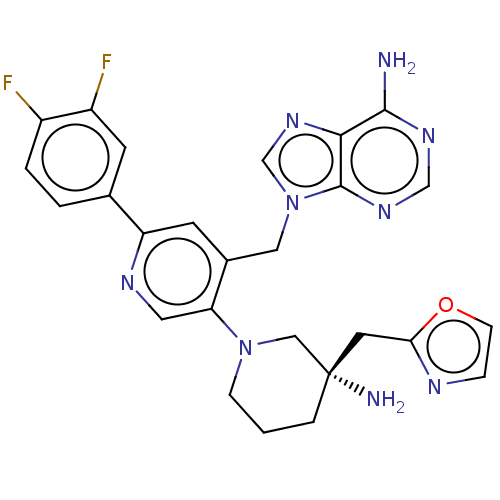 (US11420970, Example 52 | US11420970, Example 63) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay employed LC-MS/MS technology to monitor the SAH production from the NSD2 enzymatic reaction. The enzymatic reaction was performed in white... | Citation and Details BindingDB Entry DOI: 10.7270/Q27084N0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| << First | Previous | Displayed 51 to 100 (of 609 total ) | Next | Last >> |