

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM10613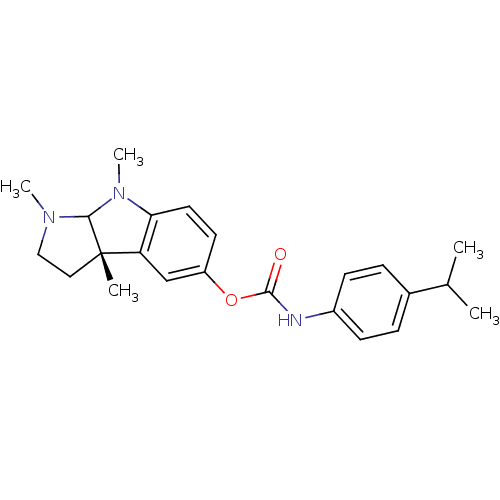 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Intramural Research Program Curated by ChEMBL | Assay Description Ability to inhibit butyrylcholinesterase (BChE), freshly prepared from human plasma | J Med Chem 42: 1855-61 (1999) Article DOI: 10.1021/jm980459s BindingDB Entry DOI: 10.7270/Q22N51GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibitory concentration against human plasma Butyrylcholinesterase | J Med Chem 48: 986-94 (2005) Article DOI: 10.1021/jm049309+ BindingDB Entry DOI: 10.7270/Q2TM7BWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibition of human plasma BChE pretreated for 30 mins by Ellman technique | Bioorg Med Chem 18: 4687-93 (2011) Article DOI: 10.1016/j.bmc.2010.05.022 BindingDB Entry DOI: 10.7270/Q2KS6SHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Intramural Research Program Curated by ChEMBL | Assay Description Ability to inhibit acetylcholinesterase (AChE), freshly prepared from human erythrocytes | J Med Chem 42: 1855-61 (1999) Article DOI: 10.1021/jm980459s BindingDB Entry DOI: 10.7270/Q22N51GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman technique | Bioorg Med Chem 18: 4687-93 (2011) Article DOI: 10.1016/j.bmc.2010.05.022 BindingDB Entry DOI: 10.7270/Q2KS6SHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibitory concentration against human erythrocyte Acetylcholinesterase | J Med Chem 48: 986-94 (2005) Article DOI: 10.1021/jm049309+ BindingDB Entry DOI: 10.7270/Q2TM7BWQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10613 ((3aS)-1,3a,8-trimethyl-1H,2H,3H,3aH,8H,8aH-pyrrolo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 8.0 | 37 |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||