

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622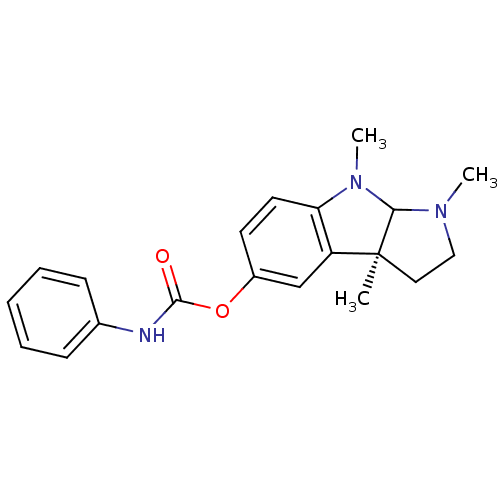 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Intramural Research Program Curated by ChEMBL | Assay Description Ability to inhibit acetylcholinesterase (AChE), freshly prepared from human erythrocytes | J Med Chem 42: 1855-61 (1999) Article DOI: 10.1021/jm980459s BindingDB Entry DOI: 10.7270/Q22N51GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibition of human whole RBC AChE pretreated for 30 mins by Ellman technique | Bioorg Med Chem 18: 4687-93 (2011) Article DOI: 10.1016/j.bmc.2010.05.022 BindingDB Entry DOI: 10.7270/Q2KS6SHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibition of human acetylcholinesterase from erythrocytes (RBC) | J Med Chem 40: 2895-901 (1997) Article DOI: 10.1021/jm970210v BindingDB Entry DOI: 10.7270/Q20V8BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of North Carolina at Chapel Hill | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 41: 2371-9 (1998) Article DOI: 10.1021/jm9800494 BindingDB Entry DOI: 10.7270/Q2W0944J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of AChE in Wistar rat brain homogenate using acetylthiocholine iodide as substrate after 0.5 hrs by Ellman's method | Bioorg Med Chem 20: 4901-14 (2012) Article DOI: 10.1016/j.bmc.2012.06.048 BindingDB Entry DOI: 10.7270/Q2W66MVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard University Curated by ChEMBL | Assay Description Inhibition acetylcholinesterase (AChE) enzyme. | J Med Chem 40: 4360-71 (1998) Article DOI: 10.1021/jm970488n BindingDB Entry DOI: 10.7270/Q2BZ6784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina Curated by ChEMBL | Assay Description Inhibitory activity against Acetylcholinesterase | J Med Chem 39: 5064-71 (1997) Article DOI: 10.1021/jm950771r BindingDB Entry DOI: 10.7270/Q2PZ59GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibition of human Butyrylcholinesterase | J Med Chem 40: 2895-901 (1997) Article DOI: 10.1021/jm970210v BindingDB Entry DOI: 10.7270/Q20V8BW2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 41: 2371-9 (1998) Article DOI: 10.1021/jm9800494 BindingDB Entry DOI: 10.7270/Q2W0944J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Intramural Research Program Curated by ChEMBL | Assay Description Ability to inhibit butyrylcholinesterase (BChE), freshly prepared from human plasma | J Med Chem 42: 1855-61 (1999) Article DOI: 10.1021/jm980459s BindingDB Entry DOI: 10.7270/Q22N51GC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 49: 2174-85 (2006) Article DOI: 10.1021/jm050578p BindingDB Entry DOI: 10.7270/Q2S75DJ6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Aging Curated by ChEMBL | Assay Description Inhibition of human plasma BChE pretreated for 30 mins by Ellman technique | Bioorg Med Chem 18: 4687-93 (2011) Article DOI: 10.1016/j.bmc.2010.05.022 BindingDB Entry DOI: 10.7270/Q2KS6SHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM10622 ((-)-Phenserine | (3aS)-1,3a,8-trimethyl-1H,2H,3H,3...) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Meiji Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of BuChE in Wistar rat plasma using acetylthiocholine iodide as substrate after 0.5 hrs by Ellman's method | Bioorg Med Chem 20: 4901-14 (2012) Article DOI: 10.1016/j.bmc.2012.06.048 BindingDB Entry DOI: 10.7270/Q2W66MVP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||