

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106736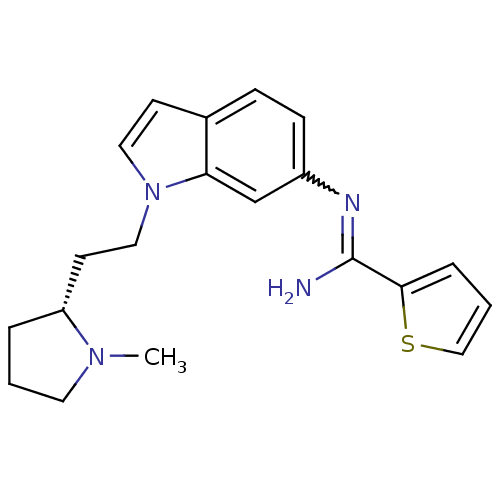 (CHEMBL1823365 | US8586620, 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant nNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human nNOS expressed in baculovirus infected insect Sf9 cells assessed as conversion of [3H]-L-arginine into [3H]-L-citrull... | Bioorg Med Chem Lett 21: 5234-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.042 BindingDB Entry DOI: 10.7270/Q2JD4X63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon, Inc. US Patent | Assay Description Enzyme assay using recombinant human inducible NOS (iNOS), human endothelial constitutive NOS (eNOS) or human neuronal constitutive NOS (nNOS). | US Patent US8586620 (2013) BindingDB Entry DOI: 10.7270/Q2348J0K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human eNOS expressed in baculovirus infected insect Sf9 cells assessed as conversion of [3H]-L-arginine into [3H]-L-citrull... | Bioorg Med Chem Lett 21: 5234-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.042 BindingDB Entry DOI: 10.7270/Q2JD4X63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant eNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS expressed in Sf9 cells assessed as inhibition of conversion of [3H]-L-arginine to [3H]-L-citrulline after 45 min... | J Med Chem 54: 7408-16 (2011) Article DOI: 10.1021/jm201063u BindingDB Entry DOI: 10.7270/Q2JQ1201 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM106736 (CHEMBL1823365 | US8586620, 138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
NeurAxon Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant human iNOS expressed in baculovirus infected insect Sf9 cells assessed as conversion of [3H]-L-arginine into [3H]-L-citrull... | Bioorg Med Chem Lett 21: 5234-8 (2011) Article DOI: 10.1016/j.bmcl.2011.07.042 BindingDB Entry DOI: 10.7270/Q2JD4X63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||