

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10890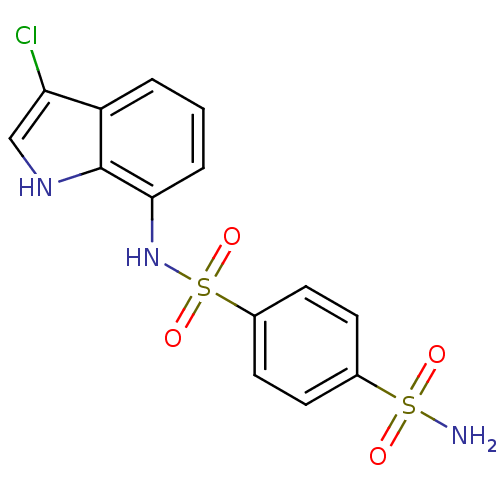 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | 80 | n/a | n/a | n/a | n/a |
GPC Biotech Incorporated | Assay Description CAII assays were performed at CEREP (paris). All other kinase assays were performed at Upstate (Dundee, UK). | Chem Biol 13: 711-22 (2006) Article DOI: 10.1016/j.chembiol.2006.05.008 BindingDB Entry DOI: 10.7270/Q2WM1BT5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Bruker-AXS s.r.l. Curated by ChEMBL | Assay Description Inhibitory activity of compound against human carbonic anhydrase II | Bioorg Med Chem Lett 14: 337-41 (2003) BindingDB Entry DOI: 10.7270/Q2W66MBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Bruker-AXS s.r.l. Curated by ChEMBL | Assay Description Inhibitory activity of compound against human carbonic anhydrase I | Bioorg Med Chem Lett 14: 337-41 (2003) BindingDB Entry DOI: 10.7270/Q2W66MBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Bruker-AXS s.r.l. Curated by ChEMBL | Assay Description Inhibitory activity of compound against bovine carbonic anhydrase IV | Bioorg Med Chem Lett 14: 337-41 (2003) BindingDB Entry DOI: 10.7270/Q2W66MBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Similar to alpha-tubulin isoform 1 (Bos taurus) | BDBM10890 (1-N-(3-chloro-1H-indol-7-yl)benzene-1,4-disulfonam...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Inhibitory activity against tubulin polymerization. | J Med Chem 45: 4913-22 (2002) Article DOI: 10.1021/jm0201060 BindingDB Entry DOI: 10.7270/Q2668GZX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||