

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macrophage metalloelastase (Homo sapiens (Human)) | BDBM120155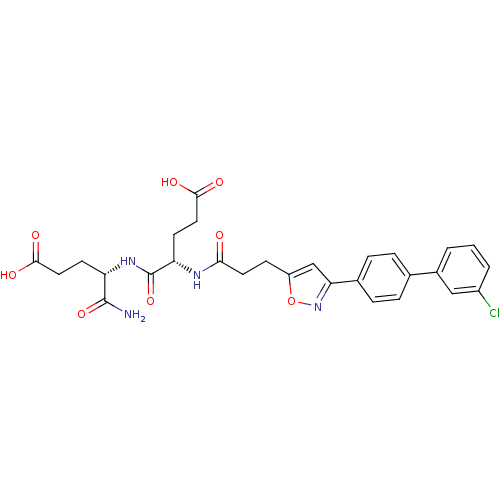 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB US Patent | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-2 (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 181 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | 565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 [1-20,P8S] (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | MMDB Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM120155 (US8691753, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | US Patent | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Commissariat A l'Energie Atomique et Aux Energies Alternatives US Patent | Assay Description The inhibition tests and the evaluation of the inhibition constants (Ki) on the various MMPs were carried out as described by Devel et al. (Devel et ... | US Patent US8691753 (2014) BindingDB Entry DOI: 10.7270/Q28P5Z5Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||