

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043760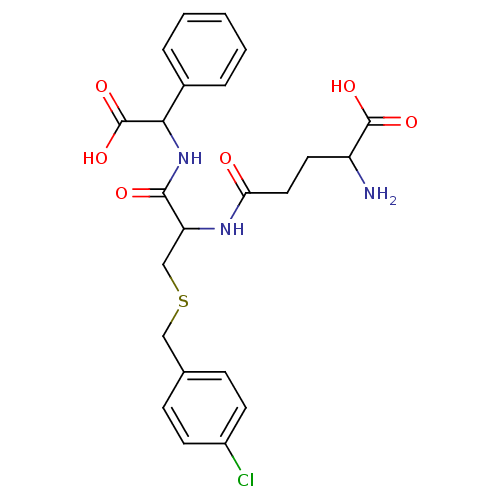 (2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50562986 (CHEMBL4757438) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of GSTP1-1 (unknown origin) assessed as inhibition constant using reduced GSH and CDNB as substrate by spectrophotometric m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02048 BindingDB Entry DOI: 10.7270/Q27948D3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043762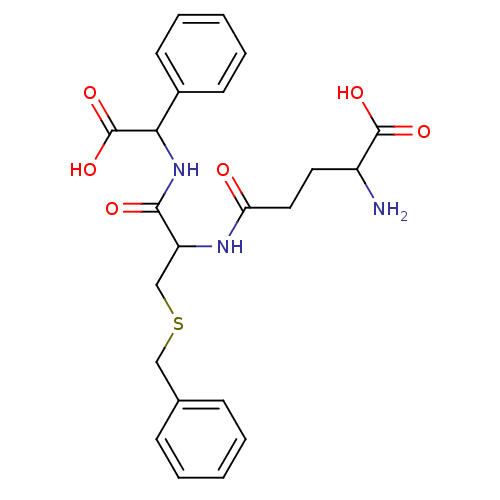 (2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50173725 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(2-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università della Magna Graecia Curated by ChEMBL | Assay Description Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzene | J Med Chem 48: 6084-9 (2005) Article DOI: 10.1021/jm0504609 BindingDB Entry DOI: 10.7270/Q22R3R6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50173727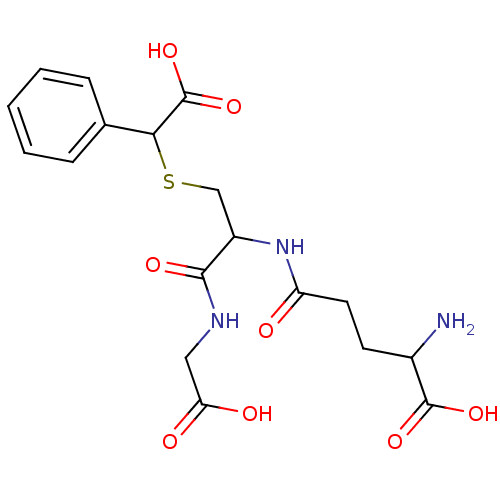 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(carboxy-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università della Magna Graecia Curated by ChEMBL | Assay Description Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzene | J Med Chem 48: 6084-9 (2005) Article DOI: 10.1021/jm0504609 BindingDB Entry DOI: 10.7270/Q22R3R6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043764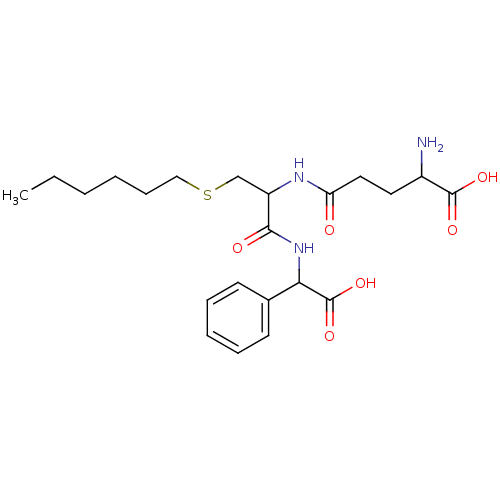 (2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50173728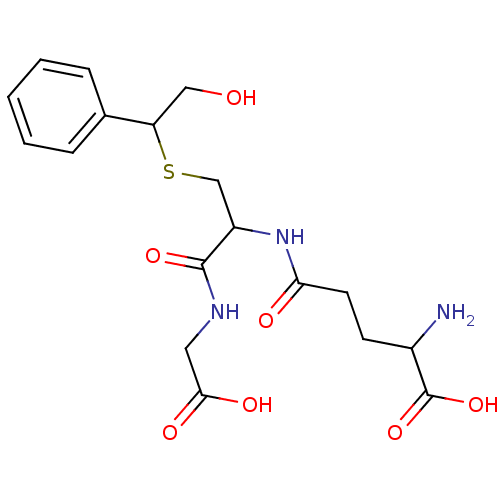 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(2-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università della Magna Graecia Curated by ChEMBL | Assay Description Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzene | J Med Chem 48: 6084-9 (2005) Article DOI: 10.1021/jm0504609 BindingDB Entry DOI: 10.7270/Q22R3R6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50038325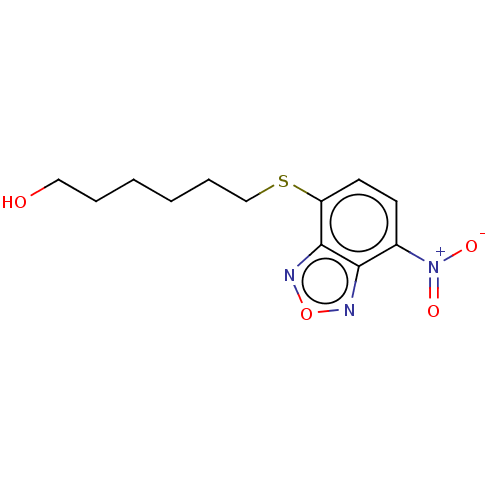 (CHEMBL1234570) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Time dependent inhibition of GSTP1-1 (unknown origin) assessed as inhibition constant using reduced GSH and CDNB as substrate by spectrophotometric m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c02048 BindingDB Entry DOI: 10.7270/Q27948D3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50148144 (5-[1-Bromo-meth-(E)-ylidene]-3-((E)-styryl)-dihydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity of the compound for human Glutathione S-transferase P was determined | J Med Chem 47: 3282-94 (2004) Article DOI: 10.1021/jm0499615 BindingDB Entry DOI: 10.7270/Q29C6WWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043758 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043763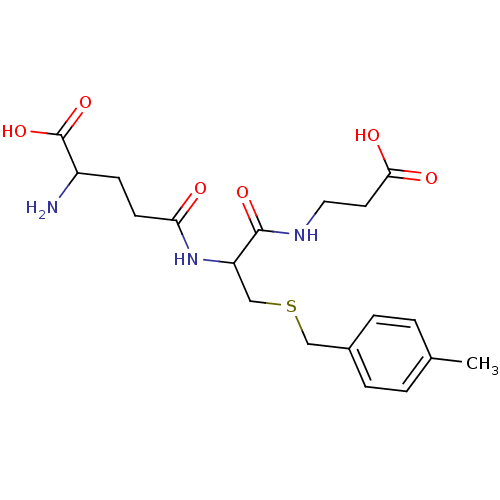 (2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50148143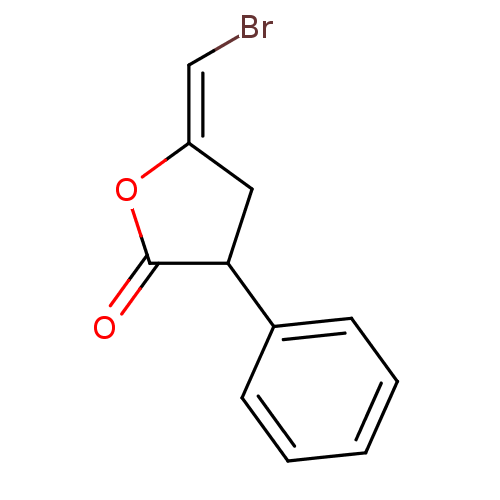 (5-[1-Bromo-meth-(E)-ylidene]-3-phenyl-dihydro-fura...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.24E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibitory activity of the compound for human glutathione S-transferase-pi isozyme was determined | J Med Chem 47: 3282-94 (2004) Article DOI: 10.1021/jm0499615 BindingDB Entry DOI: 10.7270/Q29C6WWW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50173726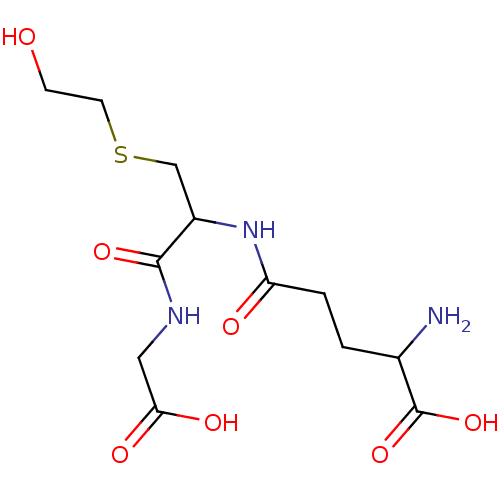 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-(2-hydrox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Università della Magna Graecia Curated by ChEMBL | Assay Description Inhibition constant against glutathione S-transferase pi using GSH (0.1-3mM), 1 mM 1-chloro-2,4-dinitrobenzene | J Med Chem 48: 6084-9 (2005) Article DOI: 10.1021/jm0504609 BindingDB Entry DOI: 10.7270/Q22R3R6R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043761 (2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase P (Homo sapiens (Human)) | BDBM50043759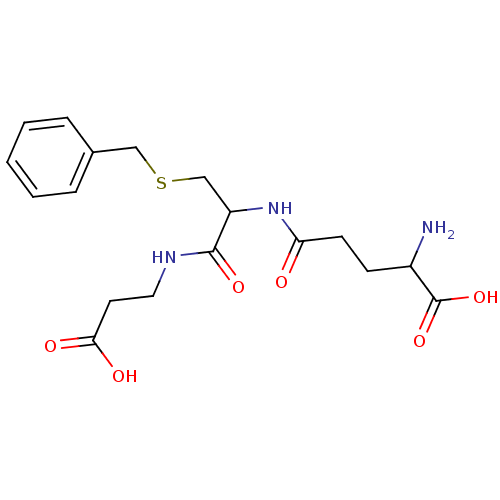 (2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase P | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||