

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aromatase (Homo sapiens (Human)) | BDBM9991 ((2S,6S,15S)-6-hydroxy-2-(methoxymethyl)-15-methylt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
Tohoku Pharmaceutical University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H] Androstenedione during aromatization. After incubation, the re... | J Med Chem 44: 4277-83 (2001) Article DOI: 10.1021/jm010282t BindingDB Entry DOI: 10.7270/Q2SB43ZH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24303 ((2-methoxy-4-{[3-methoxy-4-(sulfamoyloxy)phenyl](1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 51: 4226-38 (2008) Article DOI: 10.1021/jm800168s BindingDB Entry DOI: 10.7270/Q26W98DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9467 (1-(9-Phenyl-9H-fluoren-9-yl)-1H-1,2,3-triazole | F...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9474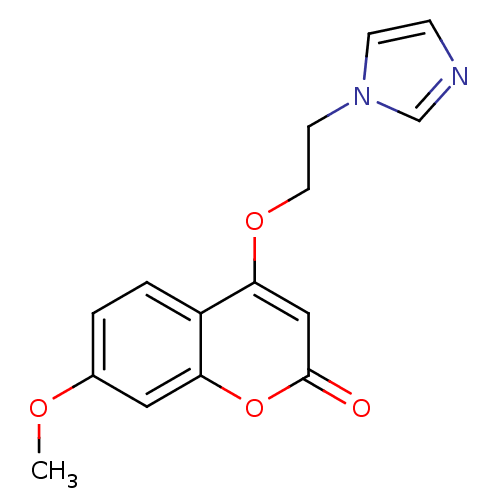 (4-[2-(1H-Imidazol-1-yl)ethoxy]-7-methoxy-2H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9479 (7-(1H-1,2,4-Triazol-1-ylmethyl)-2H-chromen-2-one |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9480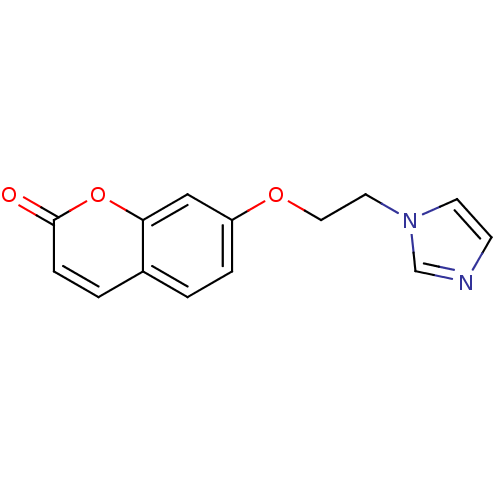 (7-[2-(1H-Imidazol-1-yl)ethoxy]-2H-chromen-2-one | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9481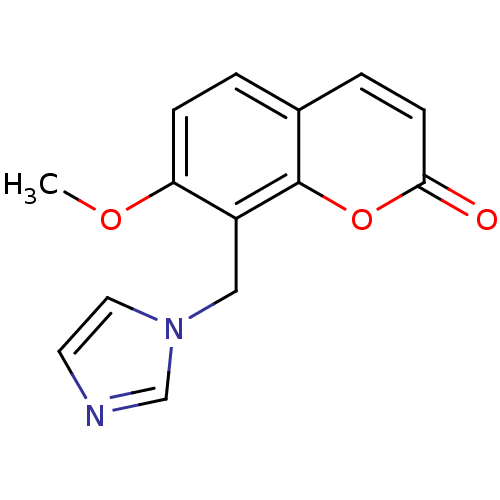 (8-(1H-Imidazol-1-ylmethyl)-7-methoxy-2H-chromen-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9484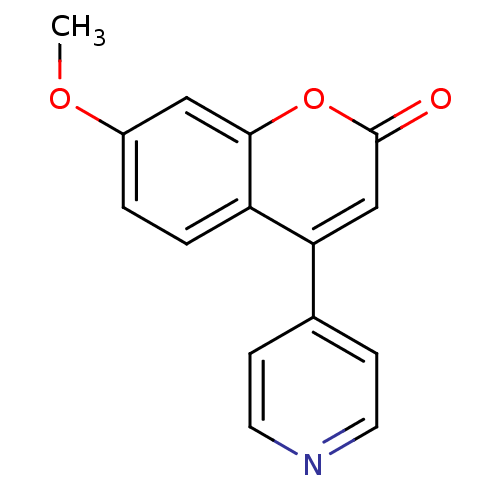 (7-methoxy-4-(pyridin-4-yl)-2H-chromen-2-one | Coum...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8938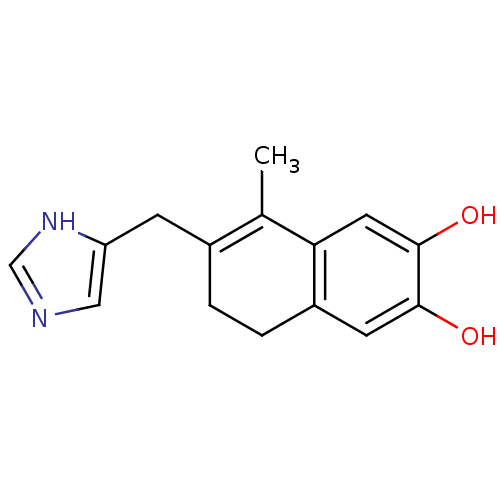 (7-(1H-Imidazol-5-ylmethyl)-8-methyl-5,6-dihydro-na...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8939 (4-(5-Methoxy-7b-methyl-3,7b-dihydro-2H-1-oxa-cyclo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8947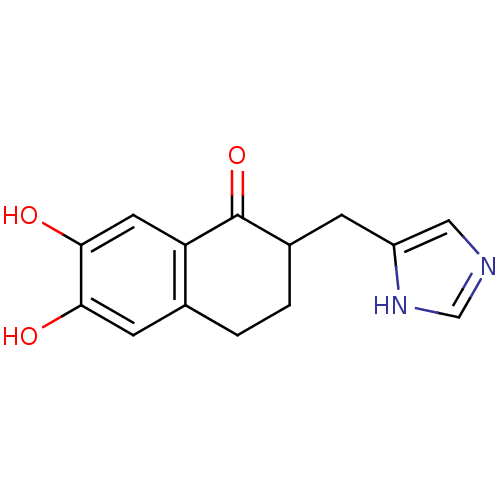 (6,7-Dihydroxy-2-(1H-imidazol-5-ylmethyl)-3,4-dihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8948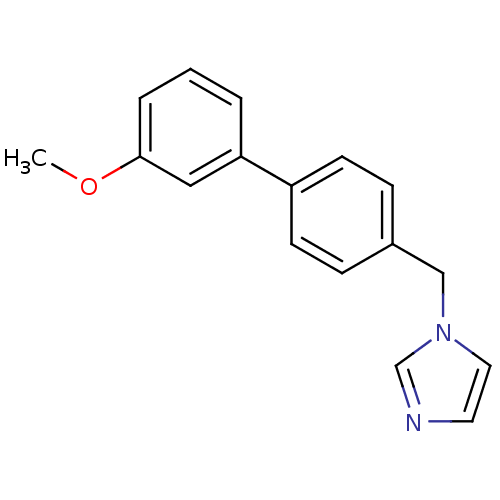 (1-{[4-(3-methoxyphenyl)phenyl]methyl}-1H-imidazole...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8949 (3-[4-(1H-imidazol-1-ylmethyl)phenyl]phenol | BW95 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8950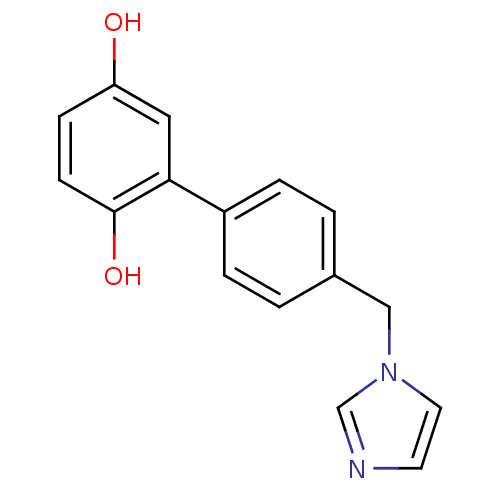 (2-[4-(1H-imidazol-1-ylmethyl)phenyl]benzene-1,4-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8952 (4-Imidazol-1-ylmethyl-biphenyl-2,3,4-triol | 4-[4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8954 (1-[(2,5-dimethoxy-1,1-biphenyl-4-yl)methyl]-1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8955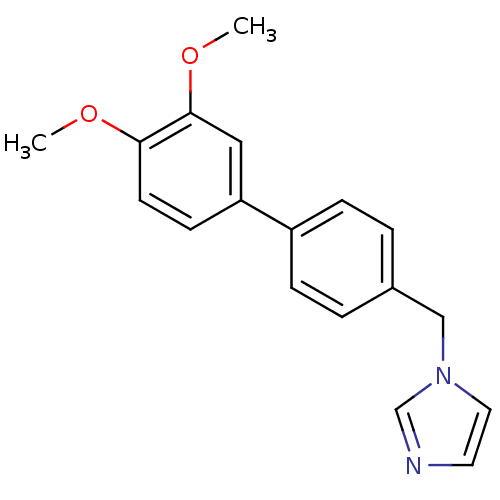 (1-[(3,4-dimethoxy-1,1-biphenyl-4-yl)methyl]-1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8957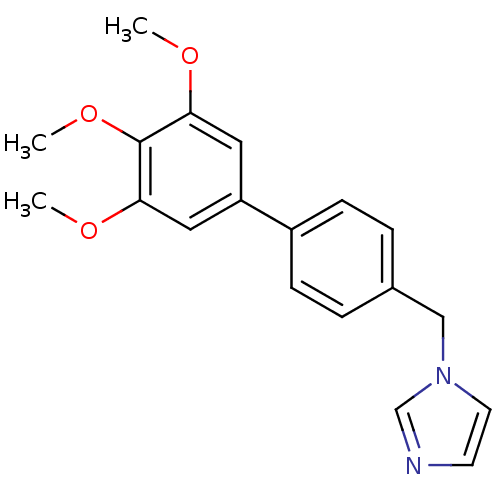 (1-[(3,4,5-trimethoxy-1,1-biphenyl-4-yl)methyl]-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Saarland University | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta-3H]androstenedione during aromatization. After incubation, the rea... | J Enzyme Inhib Med Chem 19: 17-32 (2004) Article DOI: 10.1080/14756360310001640913 BindingDB Entry DOI: 10.7270/Q2CF9N9T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM24302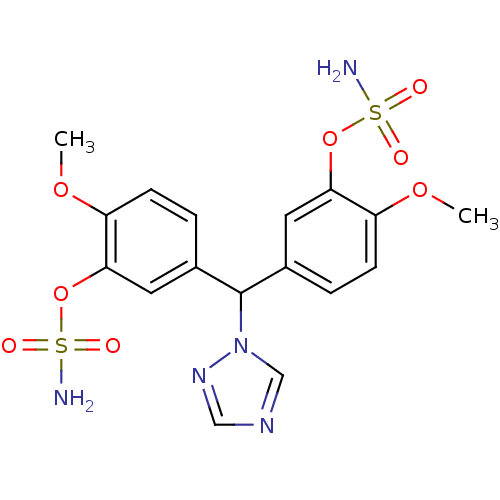 ((2-methoxy-5-{[4-methoxy-3-(sulfamoyloxy)phenyl](1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Bath | Assay Description The extent of in vitro inhibition of aromatase activities was assessed using intact monolayers of JEG-3 cells. Aromatase activity was measured using ... | J Med Chem 51: 4226-38 (2008) Article DOI: 10.1021/jm800168s BindingDB Entry DOI: 10.7270/Q26W98DJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM9466 (4-(9-Phenyl-9H-fluoren-9-yl)-4H-1,2,4-triazole | F...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
University of Bari | Assay Description The enzyme activity was assayed by measuring the 3H-labeled H2O formed from [1beta, 2beta-3H] testosterone or [1beta-3H] androstenedione during aroma... | J Med Chem 47: 6792-803 (2004) Article DOI: 10.1021/jm049535j BindingDB Entry DOI: 10.7270/Q2MS3R0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||