

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085948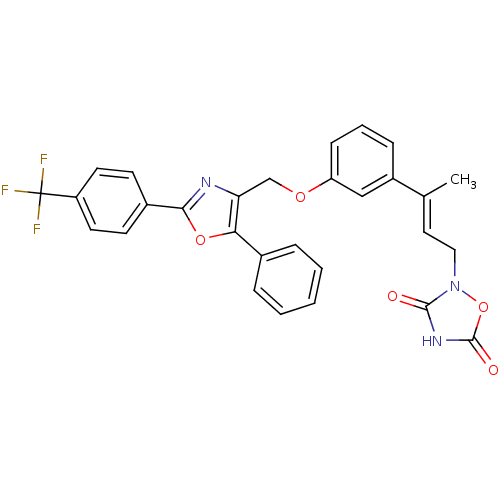 (2-(3-{3-[5-Phenyl-2-(4-trifluoromethyl-phenyl)-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085938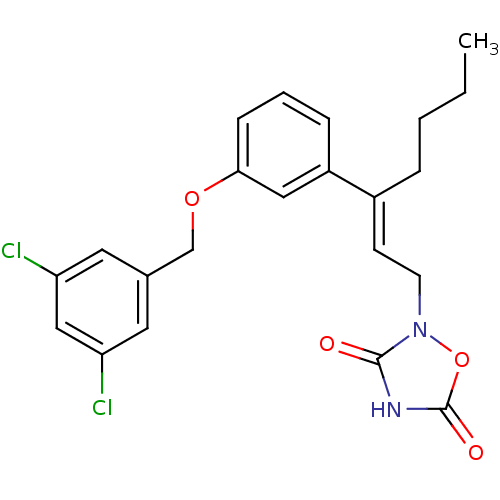 (2-{3-[3-(3,5-Dichloro-benzyloxy)-phenyl]-hept-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085965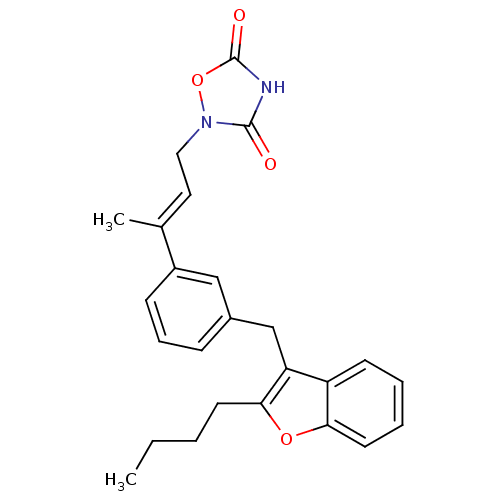 (2-{3-[3-(2-Butyl-benzofuran-3-ylmethyl)-phenyl]-bu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085942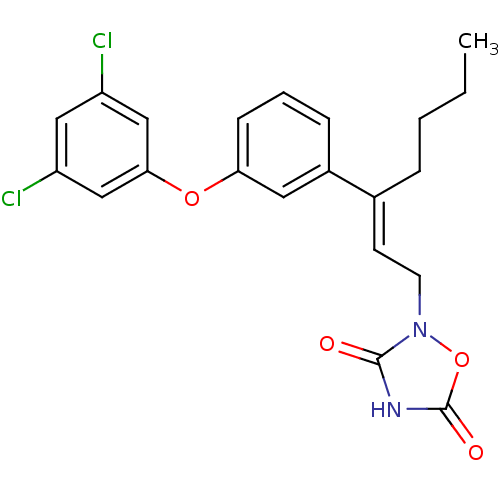 (2-{(E)-3-[3-(3,5-Dichloro-phenoxy)-phenyl]-hept-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085956 (2-[3-(4-{2-[5-Methyl-2-(4-trifluoromethyl-phenyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085938 (2-{3-[3-(3,5-Dichloro-benzyloxy)-phenyl]-hept-2-en...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant Protein-tyrosine phosphatase 1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085941 (2-[3-(3-Phenoxy-phenyl)-hept-2-enyl]-[1,2,4]oxadia...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085961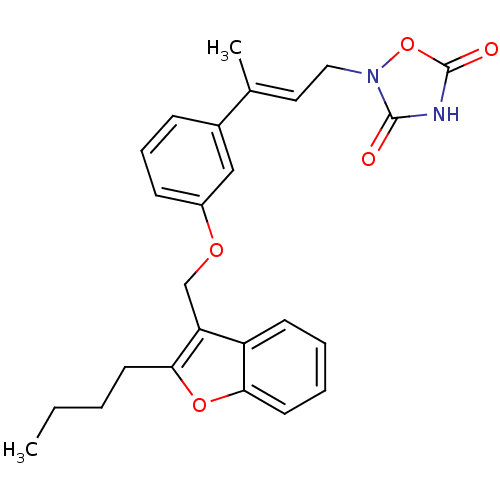 (2-{3-[3-(2-Butyl-benzofuran-3-ylmethoxy)-phenyl]-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085967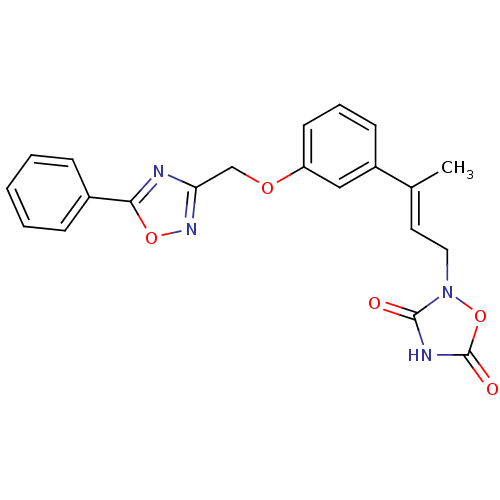 (2-{3-[3-(5-Phenyl-[1,2,4]oxadiazol-3-ylmethoxy)-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085958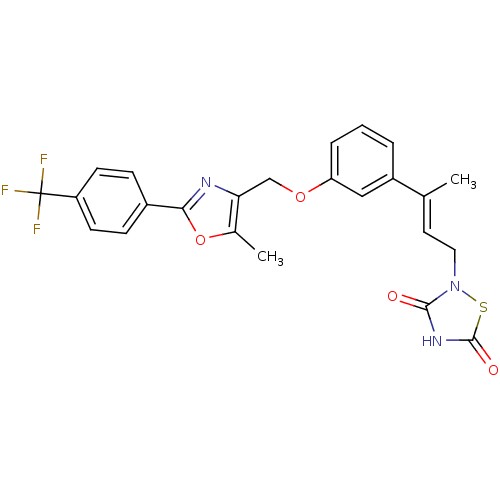 (2-(3-{3-[5-Methyl-2-(4-trifluoromethyl-phenyl)-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085960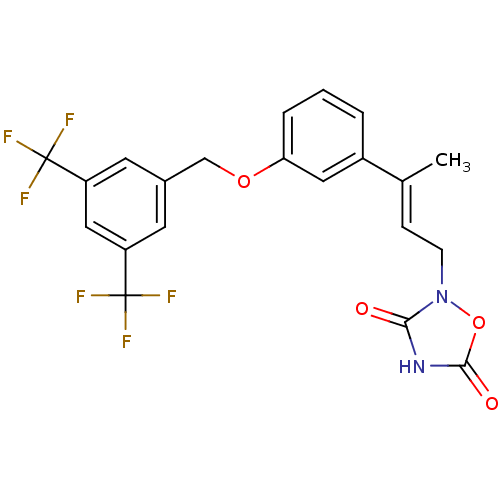 (2-{(E)-3-[3-(3,5-Bis-trifluoromethyl-benzyloxy)-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50440661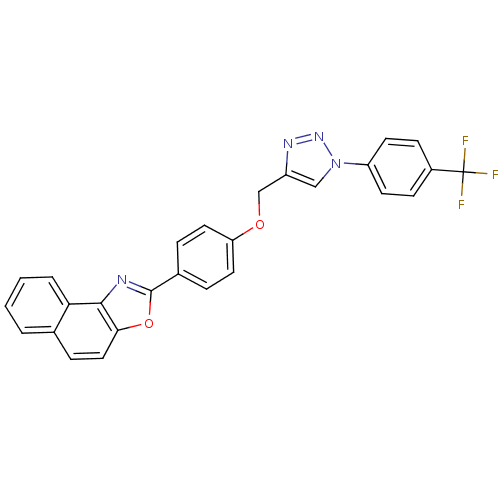 (CHEMBL2430649) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PTP1B in rat liver homogenates using p-nitro phenyl phosphate as substrate preincubated for 30 mins followed by substrate addition meas... | Citation and Details Article DOI: 10.1007/s00044-013-0784-0 BindingDB Entry DOI: 10.7270/Q2T43VJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085952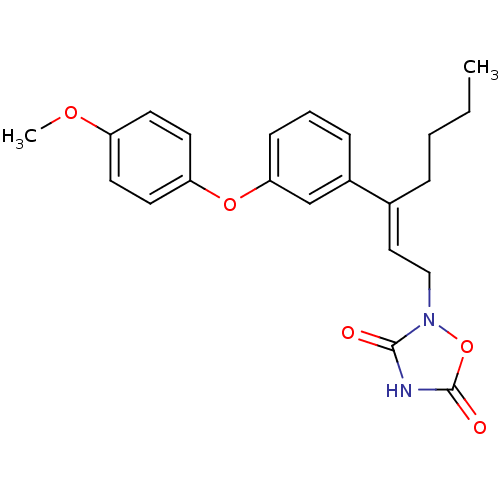 (2-{3-[3-(4-Methoxy-phenoxy)-phenyl]-hept-2-enyl}-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085963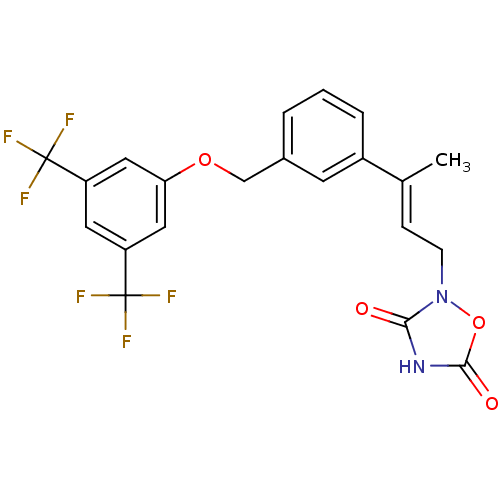 (2-{(E)-3-[3-(3,5-Bis-trifluoromethyl-phenoxymethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085939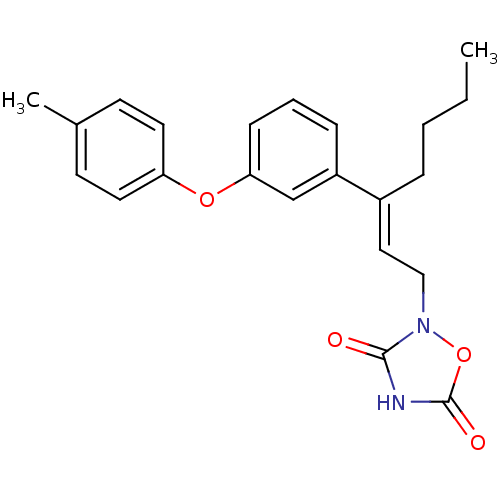 (2-[3-(3-p-Tolyloxy-phenyl)-hept-2-enyl]-[1,2,4]oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085945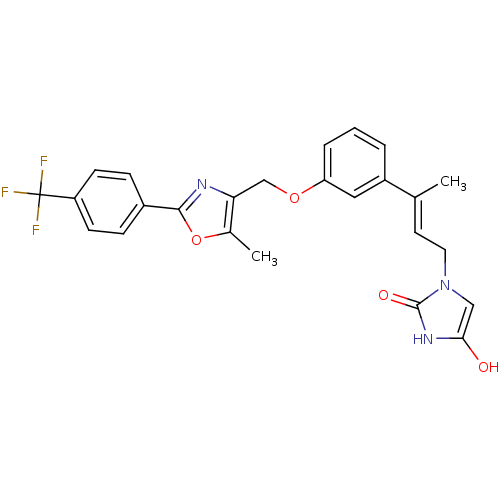 (1-(3-{3-[5-Methyl-2-(4-trifluoromethyl-phenyl)-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085955 (2-{3-[3-(5-Chloro-naphthalen-2-ylmethoxy)-phenyl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085949 (2-(3-{3-[2-(4-Trifluoromethyl-phenyl)-oxazol-4-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50440662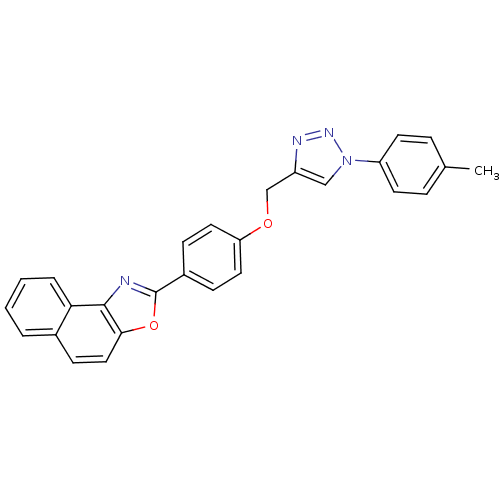 (CHEMBL2430648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of PTP1B in rat liver homogenates using p-nitro phenyl phosphate as substrate preincubated for 30 mins followed by substrate addition meas... | Citation and Details Article DOI: 10.1007/s00044-013-0784-0 BindingDB Entry DOI: 10.7270/Q2T43VJ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085942 (2-{(E)-3-[3-(3,5-Dichloro-phenoxy)-phenyl]-hept-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity in rat hepatic membrane at a concentration of 50 uM using pNPP as the substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085959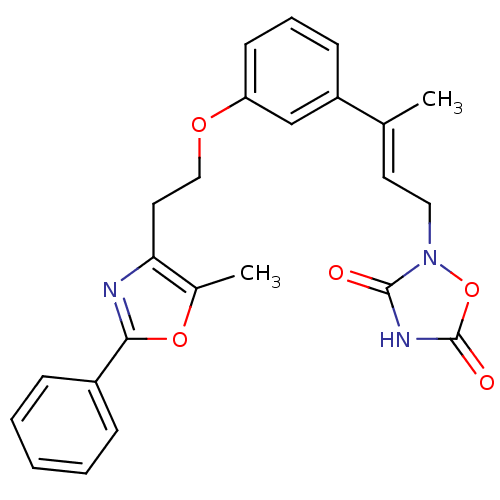 (2-(3-{3-[2-(5-Methyl-2-phenyl-oxazol-4-yl)-ethoxy]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085950 (2-(4,4-Dimethyl-3-{3-[5-methyl-2-(4-trifluoromethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity in rat hepatic membrane at a concentration of 50 uM using pNPP as the substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085969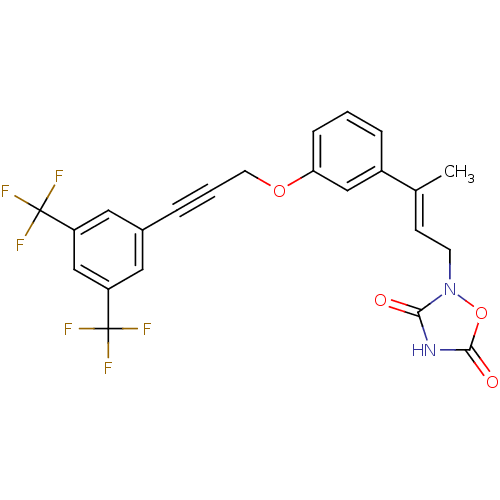 (2-(3-{3-[3-(3,5-Bis-trifluoromethyl-phenyl)-prop-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Concentration required to inhibit PTPase activity, recombinant r-PTP1B as the enzyme at a concentration 2.5 uM . | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085946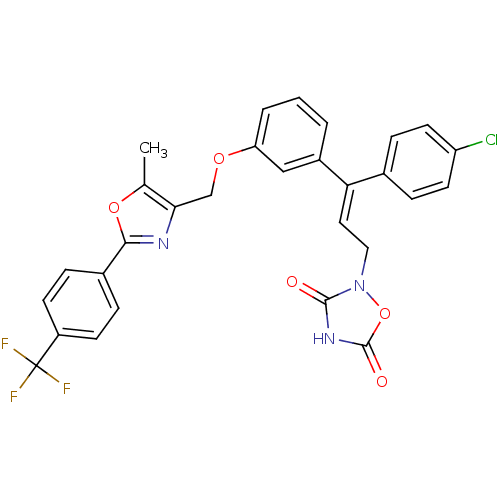 (2-(3-(4-Chloro-phenyl)-3-{3-[5-methyl-2-(4-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity in rat hepatic membrane at a concentration of 50 uM using pNPP as the substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085966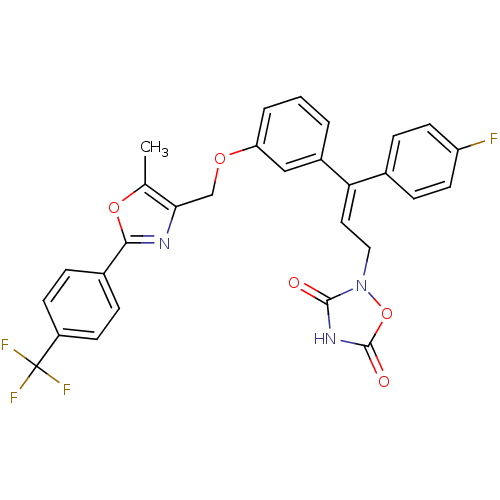 (2-(3-(4-Fluoro-phenyl)-3-{3-[5-methyl-2-(4-trifluo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity in rat hepatic membrane at a concentration of 50 uM using pNPP as the substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085961 (2-{3-[3-(2-Butyl-benzofuran-3-ylmethoxy)-phenyl]-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity in rat hepatic membrane at a concentration of 50 uM using pNPP as the substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085957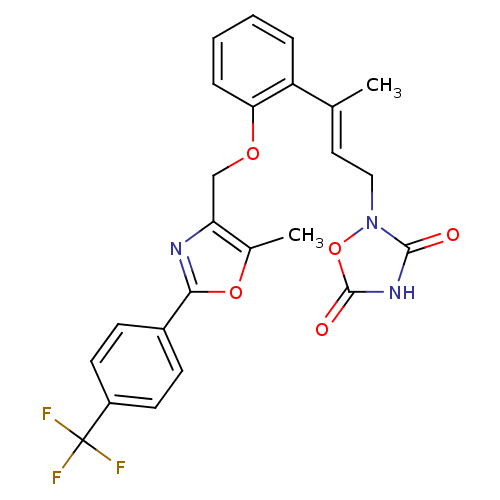 (2-(3-{2-[5-Methyl-2-(4-trifluoromethyl-phenyl)-oxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085964 (5-(3-{3-[5-Methyl-2-(4-trifluoromethoxy-phenyl)-ox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity using recombinant r-PTP1B as the enzyme and IR-triphosphopeptide as substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 1 (Rattus norvegicus (rat)) | BDBM50085940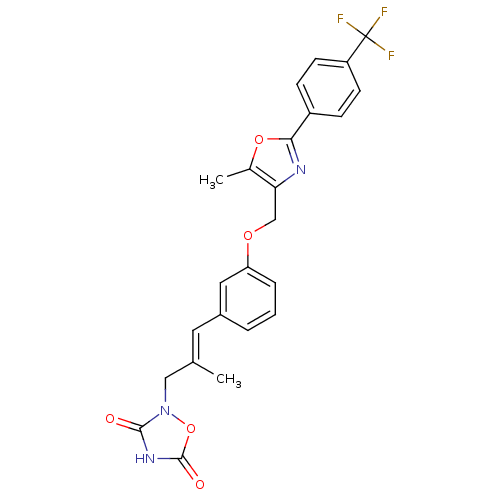 (2-(2-Methyl-3-{3-[5-methyl-2-(4-trifluoromethyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Inhibition of PTPase activity in rat hepatic membrane at a concentration of 50 uM using pNPP as the substrate | J Med Chem 43: 995-1010 (2000) BindingDB Entry DOI: 10.7270/Q2H70F1H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||