 Found 96 hits of ec50 for UniProtKB: P42338
Found 96 hits of ec50 for UniProtKB: P42338 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420309

(CHEMBL2089120)Show SMILES Cc1c(Cl)cccc1Cn1c(N)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C17H18ClN5O2S/c1-10-11(3-2-4-12(10)18)9-23-14-13(15(24)21-16(23)19)26-17(20-14)22-5-7-25-8-6-22/h2-4H,5-9H2,1H3,(H2,19,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501398

(CHEMBL4082978)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C17H14Cl2FN9O/c1-6(24-14-11(19)13(21)26-17(22)27-14)15-25-12-8(4-7(20)5-9(12)18)16(30)29(15)10-2-3-23-28-10/h2-6H,1H3,(H,23,28)(H5,21,22,24,26,27)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human ZR-75-1 cells assessed as decrease in AKT1 phosphorylation at Ser473 after 2 hrs |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501408
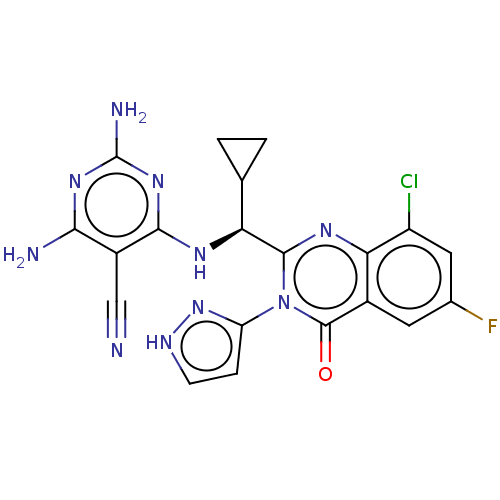
(CHEMBL4081433)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3c(Cl)cc(F)cc3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C20H16ClFN10O/c21-12-6-9(22)5-10-15(12)28-18(32(19(10)33)13-3-4-26-31-13)14(8-1-2-8)27-17-11(7-23)16(24)29-20(25)30-17/h3-6,8,14H,1-2H2,(H,26,31)(H5,24,25,27,29,30)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501405

(CHEMBL4074361)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2cccc(Cl)c2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C17H15Cl2N9O/c1-7(23-14-12(19)13(20)25-17(21)26-14)15-24-9-4-2-3-8(18)11(9)16(29)28(15)10-5-6-22-27-10/h2-7H,1H3,(H,22,27)(H5,20,21,23,25,26)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368625
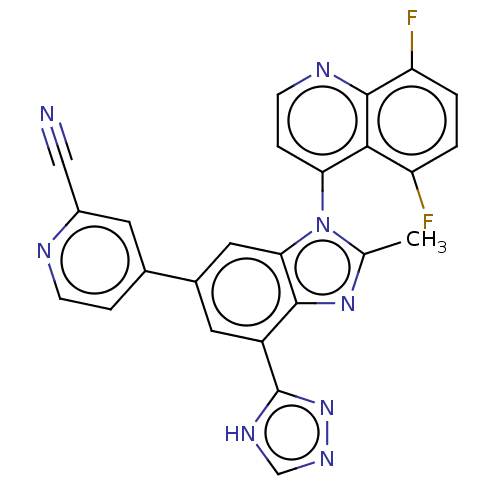
(US10227350, Compound 113 | US10227350, Compound 11...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(c1)C#N)-c1nnc[nH]1 |(-5.8,-.24,;-4.26,-.24,;-3.78,-1.7,;-2.24,-1.7,;-1.21,-2.85,;.3,-2.53,;.77,-1.06,;-.26,.08,;-1.77,-.24,;-3.01,.67,;-3.01,2.21,;-1.68,2.98,;-1.68,4.52,;-3.01,5.29,;-4.35,4.52,;-5.68,5.29,;-5.68,6.83,;-7.01,4.52,;-7.01,2.98,;-5.68,2.21,;-5.68,.67,;-4.35,2.98,;2.26,-.66,;2.66,.82,;4.14,1.22,;5.23,.13,;4.83,-1.35,;3.35,-1.75,;5.92,-2.44,;7.01,-3.53,;-1.61,-4.33,;-.7,-5.58,;-1.61,-6.83,;-3.07,-6.35,;-3.07,-4.81,)| Show InChI InChI=1S/C25H14F2N8/c1-13-33-23-17(25-31-12-32-34-25)9-15(14-4-6-29-16(8-14)11-28)10-21(23)35(13)20-5-7-30-24-19(27)3-2-18(26)22(20)24/h2-10,12H,1H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human ZR-75-1 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale ... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501398

(CHEMBL4082978)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C17H14Cl2FN9O/c1-6(24-14-11(19)13(21)26-17(22)27-14)15-25-12-8(4-7(20)5-9(12)18)16(30)29(15)10-2-3-23-28-10/h2-6H,1H3,(H,23,28)(H5,21,22,24,26,27)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human PC3 cells assessed as decrease in AKT1 phosphorylation at Ser473 after 2 hrs |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501398

(CHEMBL4082978)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C17H14Cl2FN9O/c1-6(24-14-11(19)13(21)26-17(22)27-14)15-25-12-8(4-7(20)5-9(12)18)16(30)29(15)10-2-3-23-28-10/h2-6H,1H3,(H,23,28)(H5,21,22,24,26,27)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-415 cells assessed as decrease in AKT1 phosphorylation at Ser473 after 2 hrs |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420311
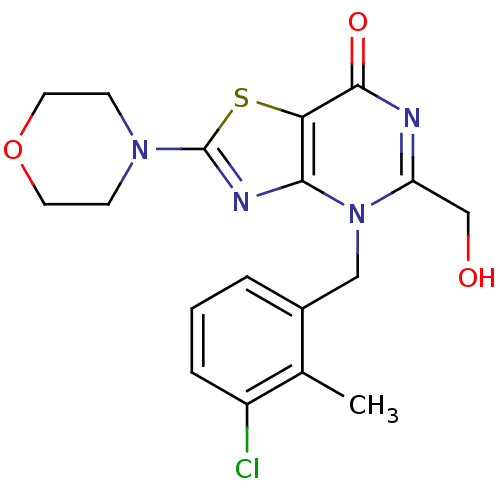
(CHEMBL2089114)Show SMILES Cc1c(Cl)cccc1Cn1c(CO)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3S/c1-11-12(3-2-4-13(11)19)9-23-14(10-24)20-17(25)15-16(23)21-18(27-15)22-5-7-26-8-6-22/h2-4,24H,5-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420310
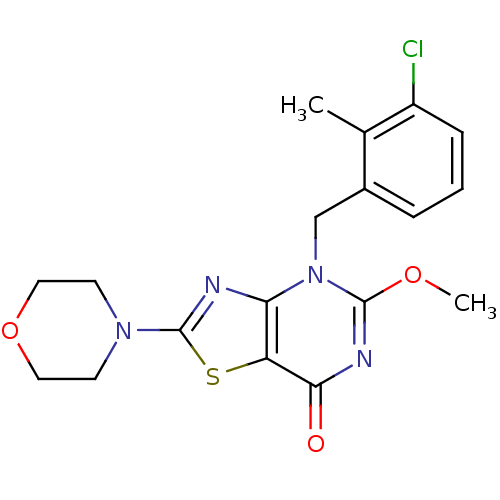
(CHEMBL2089116)Show SMILES COc1nc(=O)c2sc(nc2n1Cc1cccc(Cl)c1C)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3S/c1-11-12(4-3-5-13(11)19)10-23-15-14(16(24)21-17(23)25-2)27-18(20-15)22-6-8-26-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human ZR-75-1 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale ... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315
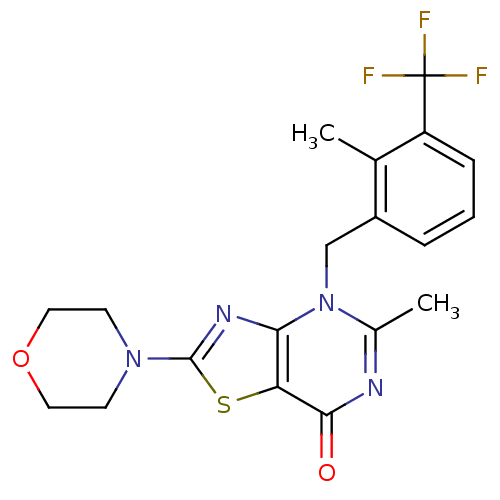
(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in absence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501398

(CHEMBL4082978)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C17H14Cl2FN9O/c1-6(24-14-11(19)13(21)26-17(22)27-14)15-25-12-8(4-7(20)5-9(12)18)16(30)29(15)10-2-3-23-28-10/h2-6H,1H3,(H,23,28)(H5,21,22,24,26,27)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCaP cells assessed as decrease in AKT1 phosphorylation at Ser473 after 2 hrs |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501410
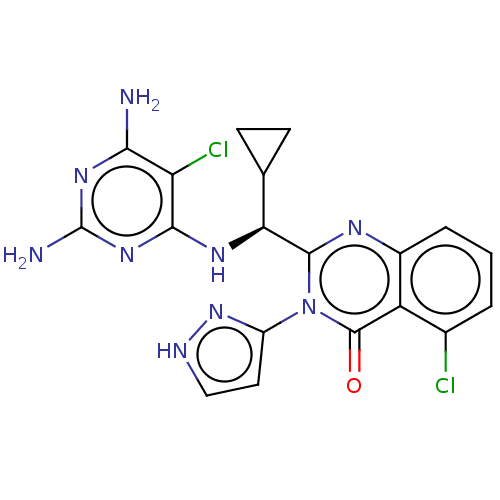
(CHEMBL4101102)Show SMILES Nc1nc(N)c(Cl)c(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)n1 |r| Show InChI InChI=1S/C19H17Cl2N9O/c20-9-2-1-3-10-12(9)18(31)30(11-6-7-24-29-11)17(25-10)14(8-4-5-8)26-16-13(21)15(22)27-19(23)28-16/h1-3,6-8,14H,4-5H2,(H,24,29)(H5,22,23,26,27,28)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human PC3 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale assa... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420312
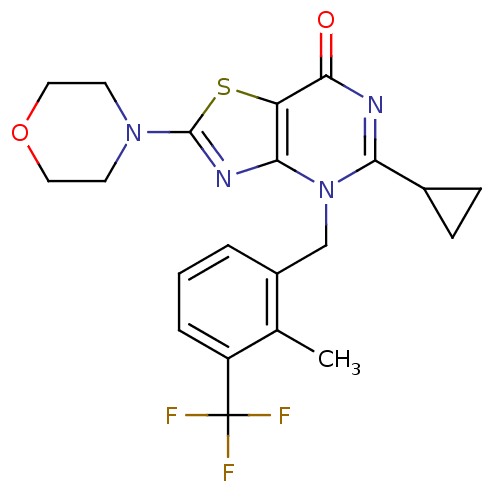
(CHEMBL2089109)Show SMILES Cc1c(Cn2c(nc(=O)c3sc(nc23)N2CCOCC2)C2CC2)cccc1C(F)(F)F Show InChI InChI=1S/C21H21F3N4O2S/c1-12-14(3-2-4-15(12)21(22,23)24)11-28-17(13-5-6-13)25-19(29)16-18(28)26-20(31-16)27-7-9-30-10-8-27/h2-4,13H,5-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-415 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesosca... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368625

(US10227350, Compound 113 | US10227350, Compound 11...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(c1)C#N)-c1nnc[nH]1 |(-5.8,-.24,;-4.26,-.24,;-3.78,-1.7,;-2.24,-1.7,;-1.21,-2.85,;.3,-2.53,;.77,-1.06,;-.26,.08,;-1.77,-.24,;-3.01,.67,;-3.01,2.21,;-1.68,2.98,;-1.68,4.52,;-3.01,5.29,;-4.35,4.52,;-5.68,5.29,;-5.68,6.83,;-7.01,4.52,;-7.01,2.98,;-5.68,2.21,;-5.68,.67,;-4.35,2.98,;2.26,-.66,;2.66,.82,;4.14,1.22,;5.23,.13,;4.83,-1.35,;3.35,-1.75,;5.92,-2.44,;7.01,-3.53,;-1.61,-4.33,;-.7,-5.58,;-1.61,-6.83,;-3.07,-6.35,;-3.07,-4.81,)| Show InChI InChI=1S/C25H14F2N8/c1-13-33-23-17(25-31-12-32-34-25)9-15(14-4-6-29-16(8-14)11-28)10-21(23)35(13)20-5-7-30-24-19(27)3-2-18(26)22(20)24/h2-10,12H,1H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human PC3 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale assa... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) after 40 mins by Kinase-Glo assay |
Eur J Med Chem 157: 37-49 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.061
BindingDB Entry DOI: 10.7270/Q2ZC85KT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501415
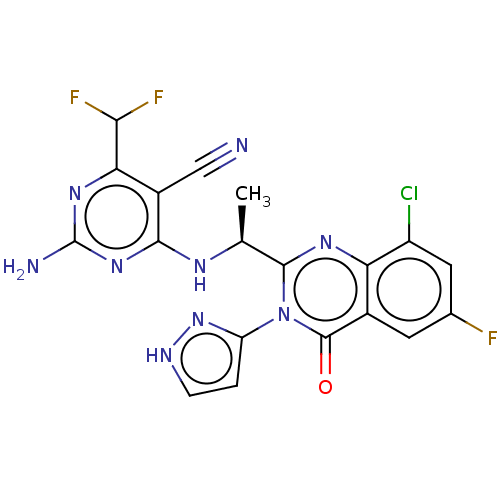
(CHEMBL4085918)Show SMILES C[C@H](Nc1nc(N)nc(C(F)F)c1C#N)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C19H13ClF3N9O/c1-7(27-16-10(6-24)14(15(22)23)29-19(25)30-16)17-28-13-9(4-8(21)5-11(13)20)18(33)32(17)12-2-3-26-31-12/h2-5,7,15H,1H3,(H,26,31)(H3,25,27,29,30)/t7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCaP C4-2 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesosca... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368625

(US10227350, Compound 113 | US10227350, Compound 11...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(c1)C#N)-c1nnc[nH]1 |(-5.8,-.24,;-4.26,-.24,;-3.78,-1.7,;-2.24,-1.7,;-1.21,-2.85,;.3,-2.53,;.77,-1.06,;-.26,.08,;-1.77,-.24,;-3.01,.67,;-3.01,2.21,;-1.68,2.98,;-1.68,4.52,;-3.01,5.29,;-4.35,4.52,;-5.68,5.29,;-5.68,6.83,;-7.01,4.52,;-7.01,2.98,;-5.68,2.21,;-5.68,.67,;-4.35,2.98,;2.26,-.66,;2.66,.82,;4.14,1.22,;5.23,.13,;4.83,-1.35,;3.35,-1.75,;5.92,-2.44,;7.01,-3.53,;-1.61,-4.33,;-.7,-5.58,;-1.61,-6.83,;-3.07,-6.35,;-3.07,-4.81,)| Show InChI InChI=1S/C25H14F2N8/c1-13-33-23-17(25-31-12-32-34-25)9-15(14-4-6-29-16(8-14)11-28)10-21(23)35(13)20-5-7-30-24-19(27)3-2-18(26)22(20)24/h2-10,12H,1H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-415 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesosca... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) after 40 mins by kinase-Glo reagent based luminescence assay |
Eur J Med Chem 158: 707-719 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.002
BindingDB Entry DOI: 10.7270/Q2PK0JVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420313

(CHEMBL2089108)Show SMILES CCc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2S/c1-3-15-24-18(28)16-17(25-19(30-16)26-7-9-29-10-8-26)27(15)11-13-5-4-6-14(12(13)2)20(21,22)23/h4-6H,3,7-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315

(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in presence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420314
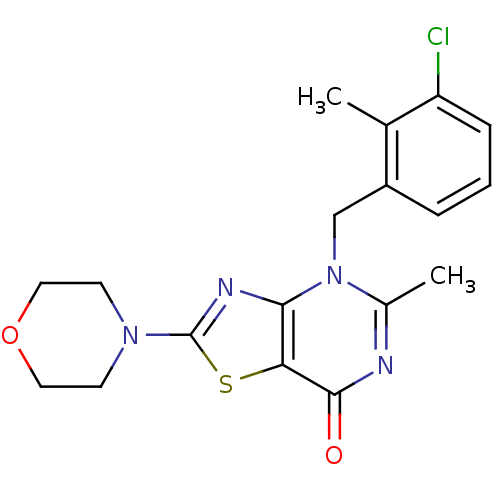
(CHEMBL2089107)Show InChI InChI=1S/C18H19ClN4O2S/c1-11-13(4-3-5-14(11)19)10-23-12(2)20-17(24)15-16(23)21-18(26-15)22-6-8-25-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501398

(CHEMBL4082978)Show SMILES C[C@H](Nc1nc(N)nc(N)c1Cl)c1nc2c(Cl)cc(F)cc2c(=O)n1-c1cc[nH]n1 |r| Show InChI InChI=1S/C17H14Cl2FN9O/c1-6(24-14-11(19)13(21)26-17(22)27-14)15-25-12-8(4-7(20)5-9(12)18)16(30)29(15)10-2-3-23-28-10/h2-6H,1H3,(H,23,28)(H5,21,22,24,26,27)/t6-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637
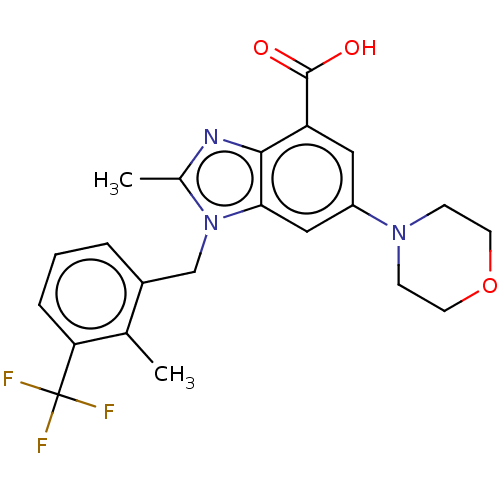
(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 23 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human PC3 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale assa... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368625

(US10227350, Compound 113 | US10227350, Compound 11...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(c1)C#N)-c1nnc[nH]1 |(-5.8,-.24,;-4.26,-.24,;-3.78,-1.7,;-2.24,-1.7,;-1.21,-2.85,;.3,-2.53,;.77,-1.06,;-.26,.08,;-1.77,-.24,;-3.01,.67,;-3.01,2.21,;-1.68,2.98,;-1.68,4.52,;-3.01,5.29,;-4.35,4.52,;-5.68,5.29,;-5.68,6.83,;-7.01,4.52,;-7.01,2.98,;-5.68,2.21,;-5.68,.67,;-4.35,2.98,;2.26,-.66,;2.66,.82,;4.14,1.22,;5.23,.13,;4.83,-1.35,;3.35,-1.75,;5.92,-2.44,;7.01,-3.53,;-1.61,-4.33,;-.7,-5.58,;-1.61,-6.83,;-3.07,-6.35,;-3.07,-4.81,)| Show InChI InChI=1S/C25H14F2N8/c1-13-33-23-17(25-31-12-32-34-25)9-15(14-4-6-29-16(8-14)11-28)10-21(23)35(13)20-5-7-30-24-19(27)3-2-18(26)22(20)24/h2-10,12H,1H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCaP C4-2 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesosca... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315

(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta in human PTEN-deficient MDA-MB-468 cells assessed as inhibition of Akt Ser473 phosphorylation |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489455
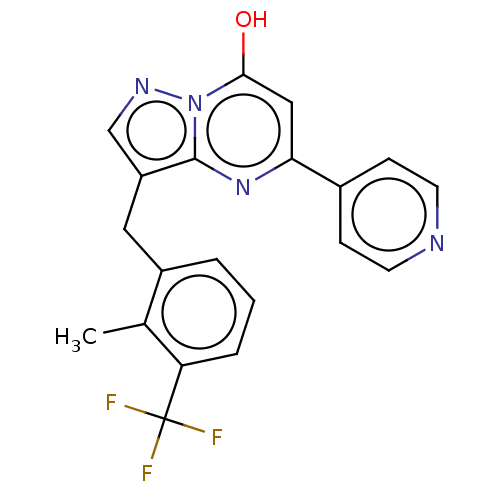
(CHEMBL2322337)Show SMILES Cc1c(Cc2cnn3c(O)cc(nc23)-c2ccncc2)cccc1C(F)(F)F Show InChI InChI=1S/C20H15F3N4O/c1-12-14(3-2-4-16(12)20(21,22)23)9-15-11-25-27-18(28)10-17(26-19(15)27)13-5-7-24-8-6-13/h2-8,10-11,28H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 25 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in absence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637

(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human ZR-75-1 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale ... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368625

(US10227350, Compound 113 | US10227350, Compound 11...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(c1)C#N)-c1nnc[nH]1 |(-5.8,-.24,;-4.26,-.24,;-3.78,-1.7,;-2.24,-1.7,;-1.21,-2.85,;.3,-2.53,;.77,-1.06,;-.26,.08,;-1.77,-.24,;-3.01,.67,;-3.01,2.21,;-1.68,2.98,;-1.68,4.52,;-3.01,5.29,;-4.35,4.52,;-5.68,5.29,;-5.68,6.83,;-7.01,4.52,;-7.01,2.98,;-5.68,2.21,;-5.68,.67,;-4.35,2.98,;2.26,-.66,;2.66,.82,;4.14,1.22,;5.23,.13,;4.83,-1.35,;3.35,-1.75,;5.92,-2.44,;7.01,-3.53,;-1.61,-4.33,;-.7,-5.58,;-1.61,-6.83,;-3.07,-6.35,;-3.07,-4.81,)| Show InChI InChI=1S/C25H14F2N8/c1-13-33-23-17(25-31-12-32-34-25)9-15(14-4-6-29-16(8-14)11-28)10-21(23)35(13)20-5-7-30-24-19(27)3-2-18(26)22(20)24/h2-10,12H,1H3,(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 31 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCAP cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale as... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489445
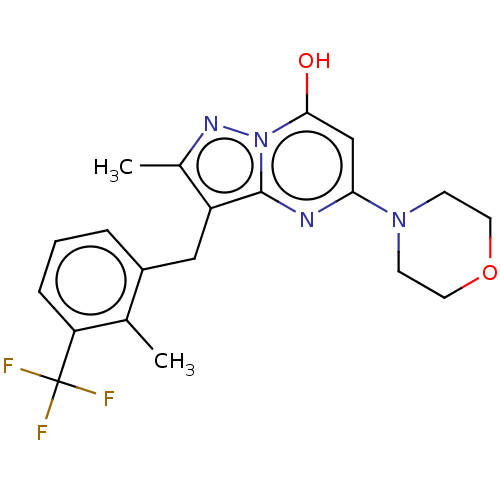
(CHEMBL2322331)Show SMILES Cc1nn2c(O)cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2/c1-12-14(4-3-5-16(12)20(21,22)23)10-15-13(2)25-27-18(28)11-17(24-19(15)27)26-6-8-29-9-7-26/h3-5,11,28H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in absence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637

(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human MDA-MB-415 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesosca... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501416
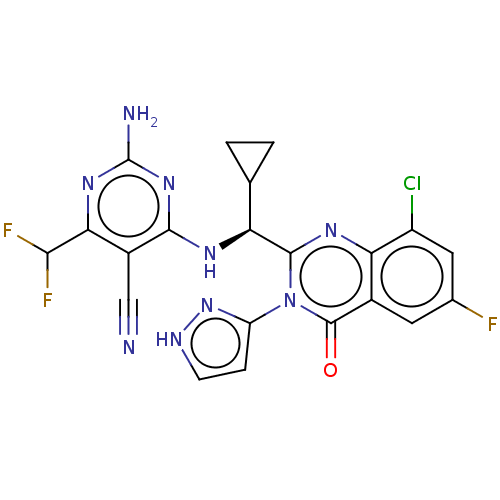
(CHEMBL4102306)Show SMILES Nc1nc(N[C@@H](C2CC2)c2nc3c(Cl)cc(F)cc3c(=O)n2-c2cc[nH]n2)c(C#N)c(n1)C(F)F |r| Show InChI InChI=1S/C21H15ClF3N9O/c22-12-6-9(23)5-10-15(12)30-19(34(20(10)35)13-3-4-28-33-13)14(8-1-2-8)29-18-11(7-26)16(17(24)25)31-21(27)32-18/h3-6,8,14,17H,1-2H2,(H,28,33)(H3,27,29,31,32)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637

(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCaP C4-2 cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesosca... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM198031
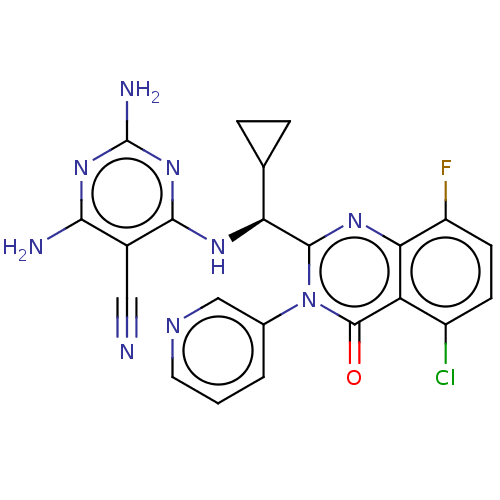
(US9221795, 27)Show SMILES Nc1nc(N)c(C#N)c(N[C@@H](C2CC2)c2nc3c(F)ccc(Cl)c3c(=O)n2-c2cccnc2)n1 |r| Show InChI InChI=1S/C22H17ClFN9O/c23-13-5-6-14(24)17-15(13)21(34)33(11-2-1-7-28-9-11)20(30-17)16(10-3-4-10)29-19-12(8-25)18(26)31-22(27)32-19/h1-2,5-7,9-10,16H,3-4H2,(H5,26,27,29,31,32)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PI3K p110beta in human PC-3 cells assessed as reduction in AKT phosphorylation at S473 residue measured after 2 hrs by TR-FRET analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b01169
BindingDB Entry DOI: 10.7270/Q20G3PV2 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50315213
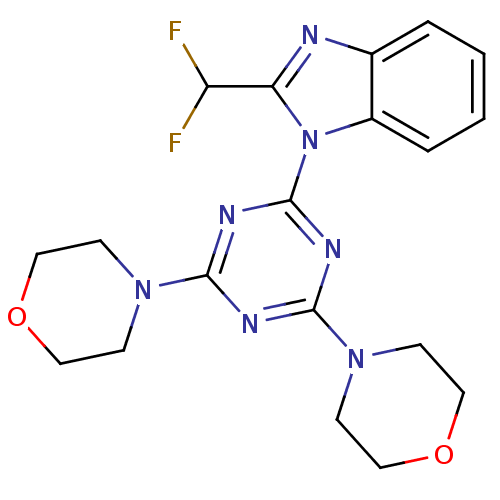
(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3K-beta (unknown origin) after 40 mins by Kinase-Glo assay |
Eur J Med Chem 157: 37-49 (2018)
Article DOI: 10.1016/j.ejmech.2018.07.061
BindingDB Entry DOI: 10.7270/Q2ZC85KT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50315213

(2-(difluoromethyl)-1-(4,6-dimorpholin-4-yl-1,3,5-t...)Show SMILES FC(F)c1nc2ccccc2n1-c1nc(nc(n1)N1CCOCC1)N1CCOCC1 Show InChI InChI=1S/C19H21F2N7O2/c20-15(21)16-22-13-3-1-2-4-14(13)28(16)19-24-17(26-5-9-29-10-6-26)23-18(25-19)27-7-11-30-12-8-27/h1-4,15H,5-12H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Chongqing University
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) after 40 mins by kinase-Glo reagent based luminescence assay |
Eur J Med Chem 158: 707-719 (2018)
Article DOI: 10.1016/j.ejmech.2018.09.002
BindingDB Entry DOI: 10.7270/Q2PK0JVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50501413
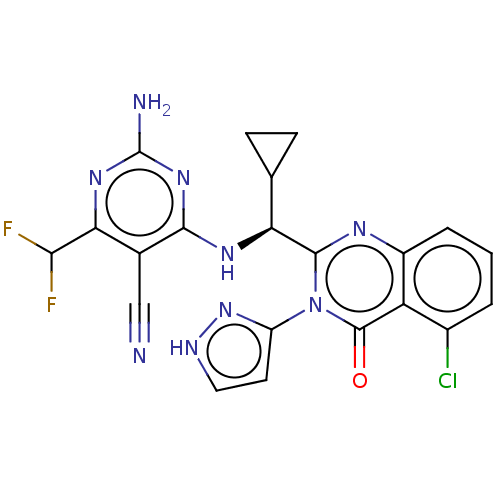
(CHEMBL4079545)Show SMILES Nc1nc(N[C@@H](C2CC2)c2nc3cccc(Cl)c3c(=O)n2-c2cc[nH]n2)c(C#N)c(n1)C(F)F |r| Show InChI InChI=1S/C21H16ClF2N9O/c22-11-2-1-3-12-14(11)20(34)33(13-6-7-27-32-13)19(28-12)15(9-4-5-9)29-18-10(8-25)16(17(23)24)30-21(26)31-18/h1-3,6-7,9,15,17H,4-5H2,(H,27,32)(H3,26,29,30,31)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 45 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110beta in PTEN-deficient human PC3 cells assessed as decrease in AKT phosphorylation at ser473 after 2 hrs by TR-FRET assay |
J Med Chem 60: 1555-1567 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01821
BindingDB Entry DOI: 10.7270/Q2BV7KNJ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM368657

(US10227350, Compound 105 | US10227350, Compound 10...)Show SMILES Cc1nc2c(cc(cc2n1-c1ccnc2c(F)ccc(F)c12)-c1ccnc(N)c1F)-c1nnc[nH]1 |(-5.25,-.24,;-3.71,-.24,;-3.24,-1.7,;-1.7,-1.7,;-.67,-2.85,;.84,-2.53,;1.32,-1.06,;.28,.08,;-1.22,-.24,;-2.47,.67,;-2.47,2.21,;-1.13,2.98,;-1.13,4.52,;-2.47,5.29,;-3.8,4.52,;-5.13,5.29,;-5.13,6.83,;-6.47,4.52,;-6.47,2.98,;-5.13,2.21,;-5.13,.67,;-3.8,2.98,;2.8,-.66,;3.2,.82,;4.69,1.22,;5.78,.13,;5.38,-1.35,;6.47,-2.44,;3.89,-1.75,;3.49,-3.24,;-1.07,-4.33,;-.16,-5.58,;-1.07,-6.83,;-2.53,-6.35,;-2.53,-4.81,)| Show InChI InChI=1S/C24H15F3N8/c1-11-33-21-14(24-31-10-32-34-24)8-12(13-4-6-30-23(28)20(13)27)9-18(21)35(11)17-5-7-29-22-16(26)3-2-15(25)19(17)22/h2-10H,1H3,(H2,28,30)(H,31,32,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 53 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCAP cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale as... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489446

(CHEMBL2322336)Show SMILES Cc1c(Cc2cnn3c(O)cc(nc23)N2CCOCC2)cccc1C(F)(F)F Show InChI InChI=1S/C19H19F3N4O2/c1-12-13(3-2-4-15(12)19(20,21)22)9-14-11-23-26-17(27)10-16(24-18(14)26)25-5-7-28-8-6-25/h2-4,10-11,27H,5-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 63 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in absence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
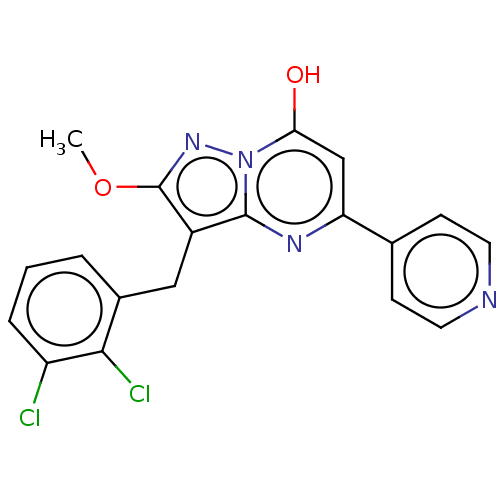
(Homo sapiens (Human)) | BDBM50489452
(CHEMBL2322344)Show SMILES COc1nn2c(O)cc(nc2c1Cc1cccc(Cl)c1Cl)-c1ccncc1 Show InChI InChI=1S/C19H14Cl2N4O2/c1-27-19-13(9-12-3-2-4-14(20)17(12)21)18-23-15(10-16(26)25(18)24-19)11-5-7-22-8-6-11/h2-8,10,26H,9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 158 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in absence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50059637

(GSK-2636771 | GSK2636771 | US10660898, Example 31)Show SMILES Cc1nc2c(cc(cc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C22H22F3N3O3/c1-13-15(4-3-5-18(13)22(23,24)25)12-28-14(2)26-20-17(21(29)30)10-16(11-19(20)28)27-6-8-31-9-7-27/h3-5,10-11H,6-9,12H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 178 | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta in PTEN-deficient human LNCAP cells assessed as reduction in AKT phosphorylation at Ser473 residue after 2 hrs by mesoscale as... |
ACS Med Chem Lett 11: 1236-1243 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00095
BindingDB Entry DOI: 10.7270/Q2K35Z7J |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489440

(CHEMBL2322349)Show SMILES Cc1nn2c(cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1)C(O)=O Show InChI InChI=1S/C21H21F3N4O3/c1-12-14(4-3-5-16(12)21(22,23)24)10-15-13(2)26-28-17(20(29)30)11-18(25-19(15)28)27-6-8-31-9-7-27/h3-5,11H,6-10H2,1-2H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in presence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489460
(CHEMBL2322332)Show InChI InChI=1S/C22H22N4O2/c1-15-19(13-17-7-4-6-16-5-2-3-8-18(16)17)22-23-20(14-21(27)26(22)24-15)25-9-11-28-12-10-25/h2-8,14,27H,9-13H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in presence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489441

(CHEMBL2322352)Show SMILES Cc1nn2c(N)cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H22F3N5O/c1-12-14(4-3-5-16(12)20(21,22)23)10-15-13(2)26-28-17(24)11-18(25-19(15)28)27-6-8-29-9-7-27/h3-5,11H,6-10,24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in presence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489446

(CHEMBL2322336)Show SMILES Cc1c(Cc2cnn3c(O)cc(nc23)N2CCOCC2)cccc1C(F)(F)F Show InChI InChI=1S/C19H19F3N4O2/c1-12-13(3-2-4-15(12)19(20,21)22)9-14-11-23-26-17(27)10-16(24-18(14)26)25-5-7-28-8-6-25/h2-4,10-11,27H,5-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in presence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
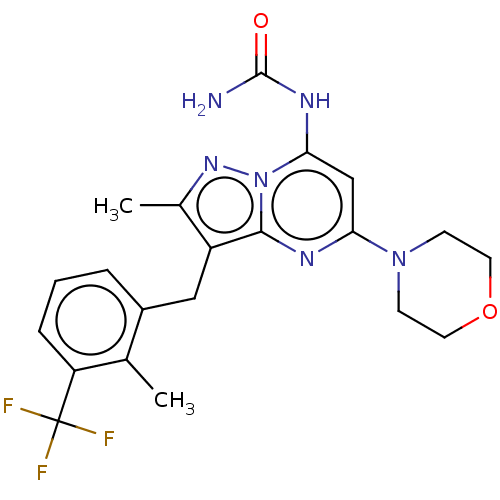
(Homo sapiens (Human)) | BDBM50489437
(CHEMBL2322356)Show SMILES Cc1nn2c(NC(N)=O)cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C21H23F3N6O2/c1-12-14(4-3-5-16(12)21(22,23)24)10-15-13(2)28-30-18(27-20(25)31)11-17(26-19(15)30)29-6-8-32-9-7-29/h3-5,11H,6-10H2,1-2H3,(H3,25,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as cell growth inhibition |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489445

(CHEMBL2322331)Show SMILES Cc1nn2c(O)cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2/c1-12-14(4-3-5-16(12)20(21,22)23)10-15-13(2)25-27-18(28)11-17(24-19(15)27)26-6-8-29-9-7-26/h3-5,11,28H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.59E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta human in PTEN-null MDA-MB-468 cells assessed as reduction of AKT phosphorylation at Ser 473 in presence of serum |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data