 Found 457 hits of ec50 data for polymerid = 2257
Found 457 hits of ec50 data for polymerid = 2257 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559603

(CHEMBL4787458)Show SMILES Cc1cc(OC2CCC3(CCCC3)CC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559624

(CHEMBL4754965)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNC2CC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(15.22,-4.91,;15.22,-6.45,;13.9,-7.23,;13.89,-8.77,;12.56,-9.54,;11.23,-8.77,;11.24,-7.22,;9.91,-6.45,;8.57,-7.21,;8.57,-8.75,;9.9,-9.53,;7.24,-6.44,;7.25,-4.9,;5.9,-7.2,;5.9,-5.66,;15.23,-9.54,;15.23,-11.08,;16.57,-11.85,;16.57,-13.39,;15.48,-14.47,;16.57,-15.56,;17.66,-14.47,;16.58,-17.1,;15.25,-17.87,;17.91,-17.87,;16.56,-8.76,;17.9,-9.53,;19.23,-8.75,;19.22,-7.21,;17.89,-6.45,;16.56,-7.22,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559628
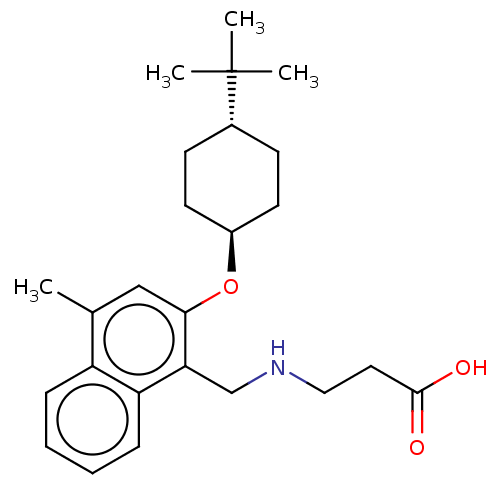
(CHEMBL4798593)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCCC(O)=O)c2ccccc12 |r,wU:5.4,wD:8.11,(15.03,-5.2,;15.04,-6.74,;13.71,-7.51,;13.71,-9.06,;12.37,-9.83,;11.04,-9.06,;9.71,-9.82,;8.38,-9.04,;8.38,-7.5,;9.72,-6.74,;11.05,-7.51,;7.05,-6.72,;7.06,-5.18,;5.72,-7.49,;5.71,-5.95,;15.04,-9.83,;15.04,-11.37,;16.38,-12.14,;17.71,-11.36,;19.05,-12.13,;20.38,-11.36,;21.72,-12.12,;20.38,-9.82,;16.37,-9.05,;17.71,-9.82,;19.04,-9.04,;19.04,-7.49,;17.7,-6.73,;16.37,-7.51,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559616
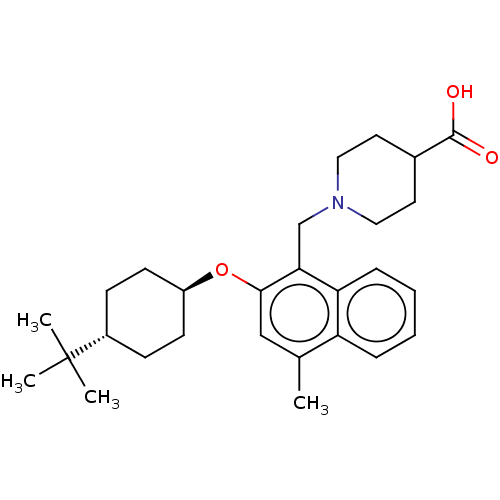
(CHEMBL4748198)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(21.06,-7.5,;21.06,-9.04,;19.74,-9.81,;19.73,-11.36,;18.4,-12.13,;17.07,-11.36,;17.07,-9.81,;15.75,-9.04,;14.41,-9.8,;14.41,-11.34,;15.74,-12.12,;13.08,-9.02,;13.09,-7.48,;11.74,-9.79,;11.74,-8.25,;21.07,-12.13,;21.07,-13.67,;22.41,-14.44,;22.4,-15.98,;23.73,-16.74,;25.07,-15.98,;25.07,-14.43,;23.73,-13.66,;26.4,-16.75,;27.74,-15.98,;26.4,-18.29,;22.4,-11.35,;23.74,-12.12,;25.07,-11.34,;25.06,-9.79,;23.72,-9.03,;22.4,-9.81,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [33P]S1P from human recombinant S1P5 receptor expressed in CHO cells by scintillation counting |
J Med Chem 53: 4198-211 (2010)
Article DOI: 10.1021/jm100181s
BindingDB Entry DOI: 10.7270/Q2513ZBQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559622
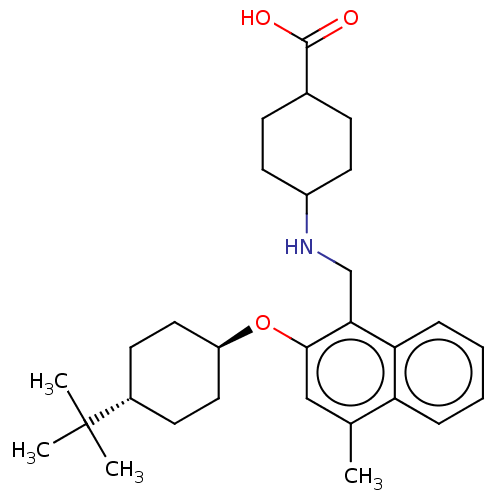
(CHEMBL4784199)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNC2CCC(CC2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(14.18,-2.77,;14.18,-4.31,;12.85,-5.08,;12.85,-6.62,;11.52,-7.39,;10.18,-6.62,;10.19,-5.07,;8.87,-4.3,;7.53,-5.06,;7.52,-6.61,;8.86,-7.39,;6.2,-4.29,;6.21,-2.75,;4.86,-5.05,;4.85,-3.52,;14.19,-7.39,;14.19,-8.93,;15.52,-9.7,;15.53,-11.24,;14.19,-12.01,;14.19,-13.54,;15.52,-14.31,;16.86,-13.54,;16.86,-12,;15.52,-15.85,;16.85,-16.63,;14.19,-16.62,;15.52,-6.62,;16.85,-7.38,;18.19,-6.61,;18.18,-5.06,;16.84,-4.3,;15.52,-5.07,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | 7.4 | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Agonism of human S1P-5 receptor expressed in CHO cells, 90-120 min in pH 7.4 using [35S]-GTP-gammaS as radioligand |
J Med Chem 48: 5373-7 (2005)
Article DOI: 10.1021/jm050242f
BindingDB Entry DOI: 10.7270/Q218361R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158336
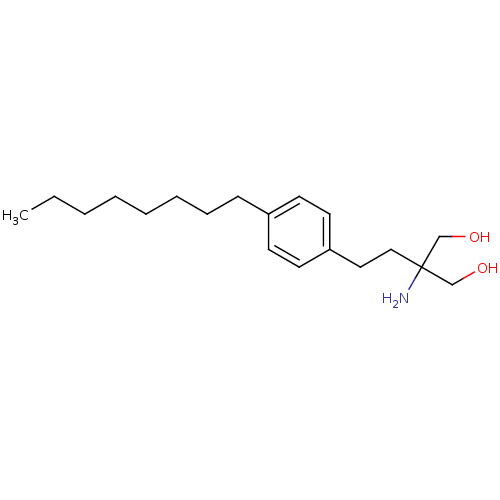
(2-(4-octylphenethyl)-2-aminopropane-1,3-diol | 2-A...)Show InChI InChI=1S/C19H33NO2/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-22/h9-12,21-22H,2-8,13-16,20H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor (unknown origin) |
Bioorg Med Chem Lett 23: 6377-89 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.058
BindingDB Entry DOI: 10.7270/Q2FR00KV |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50314401
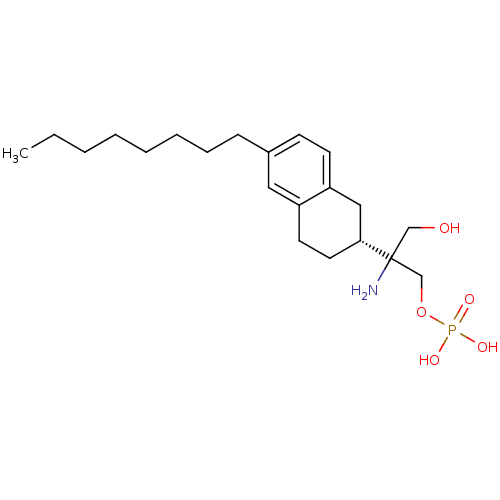
(2-amino-3-hydroxy-2-((R)-6-octyl-1,2,3,4-tetrahydr...)Show SMILES CCCCCCCCc1ccc2C[C@@H](CCc2c1)C(N)(CO)COP(O)(O)=O |r| Show InChI InChI=1S/C21H36NO5P/c1-2-3-4-5-6-7-8-17-9-10-19-14-20(12-11-18(19)13-17)21(22,15-23)16-27-28(24,25)26/h9-10,13,20,23H,2-8,11-12,14-16,22H2,1H3,(H2,24,25,26)/t20-,21?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
BiogenIdec Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor assessed as effect on calcium mobilization by Gq dependent whole cell assay |
Bioorg Med Chem Lett 20: 2264-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.006
BindingDB Entry DOI: 10.7270/Q2JS9QJS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50250631

(CHEMBL4093489)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2)c(OC)c1 |t:14| Show InChI InChI=1S/C27H33NO4/c1-4-5-19-6-7-22(26(12-19)31-3)17-32-24-10-11-25-18(2)21(9-8-20(25)13-24)14-28-15-23(16-28)27(29)30/h6-7,10-13,23H,4-5,8-9,14-17H2,1-3H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins by E... |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50314401

(2-amino-3-hydroxy-2-((R)-6-octyl-1,2,3,4-tetrahydr...)Show SMILES CCCCCCCCc1ccc2C[C@@H](CCc2c1)C(N)(CO)COP(O)(O)=O |r| Show InChI InChI=1S/C21H36NO5P/c1-2-3-4-5-6-7-8-17-9-10-19-14-20(12-11-18(19)13-17)21(22,15-23)16-27-28(24,25)26/h9-10,13,20,23H,2-8,11-12,14-16,22H2,1H3,(H2,24,25,26)/t20-,21?/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a |
BiogenIdec Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor assessed as effect on calcium mobilization by Gq dependent whole cell assay |
Bioorg Med Chem Lett 20: 2264-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.006
BindingDB Entry DOI: 10.7270/Q2JS9QJS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5R |
ACS Med Chem Lett 2: 368-372 (2011)
Article DOI: 10.1021/ml100301k
BindingDB Entry DOI: 10.7270/Q24T6JTS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
BiogenIdec Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor assessed as effect on calcium mobilization by Gq dependent whole cell assay |
Bioorg Med Chem Lett 20: 2264-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.006
BindingDB Entry DOI: 10.7270/Q2JS9QJS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as calcium flux measured for 180 secs by FLIPR assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor by GTPgammaS binding assay |
ACS Med Chem Lett 7: 283-8 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00448
BindingDB Entry DOI: 10.7270/Q2D79D95 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23164
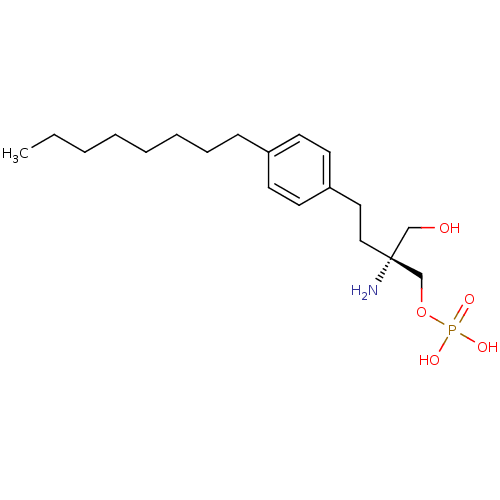
(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor expressed in CHO cells assessed as induction of [S35]GTPgammaS binding |
Bioorg Med Chem Lett 20: 1485-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.118
BindingDB Entry DOI: 10.7270/Q2WD40QR |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50532533
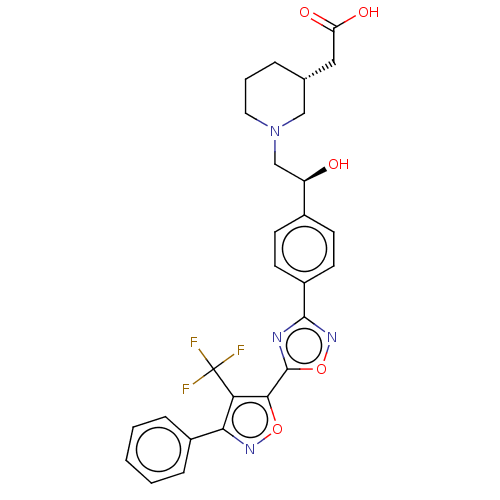
(CHEMBL4458575)Show SMILES O[C@H](CN1CCC[C@H](CC(O)=O)C1)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C27H25F3N4O5/c28-27(29,30)22-23(18-6-2-1-3-7-18)32-38-24(22)26-31-25(33-39-26)19-10-8-17(9-11-19)20(35)15-34-12-4-5-16(14-34)13-21(36)37/h1-3,6-11,16,20,35H,4-5,12-15H2,(H,36,37)/t16-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor (unknown origin) measured after 45 mins by [35S]GTP-gammaS binding assay |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50532533

(CHEMBL4458575)Show SMILES O[C@H](CN1CCC[C@H](CC(O)=O)C1)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C27H25F3N4O5/c28-27(29,30)22-23(18-6-2-1-3-7-18)32-38-24(22)26-31-25(33-39-26)19-10-8-17(9-11-19)20(35)15-34-12-4-5-16(14-34)13-21(36)37/h1-3,6-11,16,20,35H,4-5,12-15H2,(H,36,37)/t16-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor (unknown origin) measured after 45 mins by [35S]GTP-gammaS binding assay |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50313312
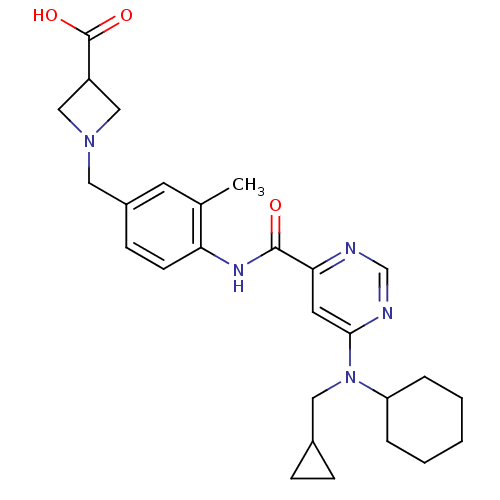
(1-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...)Show SMILES Cc1cc(CN2CC(C2)C(O)=O)ccc1NC(=O)c1cc(ncn1)N(CC1CC1)C1CCCCC1 Show InChI InChI=1S/C27H35N5O3/c1-18-11-20(13-31-15-21(16-31)27(34)35)9-10-23(18)30-26(33)24-12-25(29-17-28-24)32(14-19-7-8-19)22-5-3-2-4-6-22/h9-12,17,19,21-22H,2-8,13-16H2,1H3,(H,30,33)(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Merck Serono SA
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor expressed in CHO cells after 60 mins by [35S]-GTPgammaS binding assay |
Bioorg Med Chem Lett 20: 1516-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.102
BindingDB Entry DOI: 10.7270/Q2J38SQK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50495089
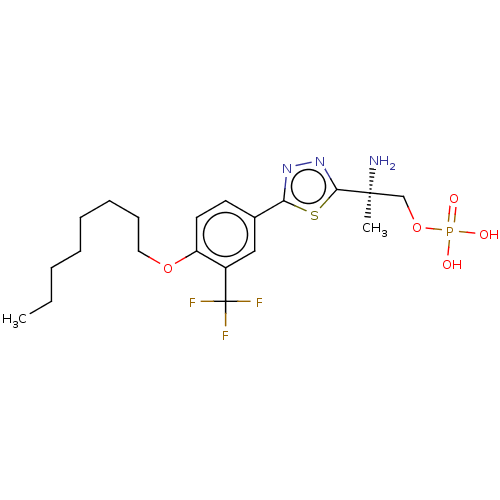
(CHEMBL3102904)Show SMILES CCCCCCCCOc1ccc(cc1C(F)(F)F)-c1nnc(s1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C20H29F3N3O5PS/c1-3-4-5-6-7-8-11-30-16-10-9-14(12-15(16)20(21,22)23)17-25-26-18(33-17)19(2,24)13-31-32(27,28)29/h9-10,12H,3-8,11,13,24H2,1-2H3,(H2,27,28,29)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5R expressed in HEK293T cells assessed as [35S]GTPgammaS binding after 30 mins by scintillation counting |
ACS Med Chem Lett 4: 942-7 (2013)
Article DOI: 10.1021/ml400194r
BindingDB Entry DOI: 10.7270/Q2RR2269 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23163

(CHEMBL114606 | FTY720-phosphate, rac-2 | {2-amino-...)Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at recombinant human S1P5 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01109
BindingDB Entry DOI: 10.7270/Q28919KX |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23165

(CHEMBL366208 | FTY720-phosphate, (S)-2 | [(2S)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human recombinant S1P5 expressed in CHO cell membranes assessed as stimulation of [35S]-GTPgammaS binding measured after 45 mins ... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.8b01695
BindingDB Entry DOI: 10.7270/Q2TQ659P |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186929

(3-(4-(5-(3-cyano-4-(1,1,1,3,3,3-hexafluoropropan-2...)Show SMILES Cc1cc(CCC(O)=O)ccc1-c1noc(n1)-c1ccc(OC(C(F)(F)F)C(F)(F)F)c(c1)C#N Show InChI InChI=1S/C22H15F6N3O4/c1-11-8-12(3-7-17(32)33)2-5-15(11)18-30-19(35-31-18)13-4-6-16(14(9-13)10-29)34-20(21(23,24)25)22(26,27)28/h2,4-6,8-9,20H,3,7H2,1H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake |
Bioorg Med Chem Lett 16: 3679-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.084
BindingDB Entry DOI: 10.7270/Q2J67GJ8 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559623
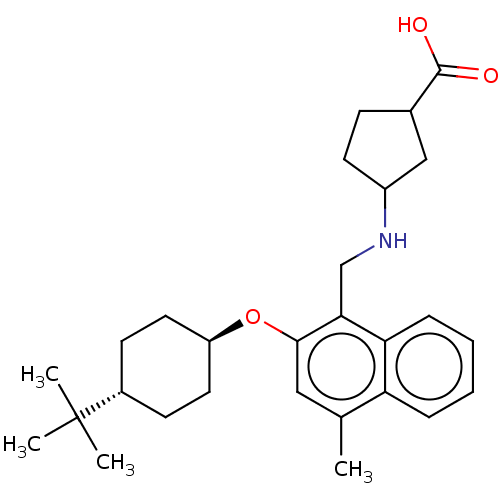
(CHEMBL4747262)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNC2CCC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(14.87,-3.29,;14.87,-4.83,;13.55,-5.6,;13.54,-7.14,;12.21,-7.91,;10.88,-7.14,;10.89,-5.59,;9.56,-4.82,;8.22,-5.59,;8.22,-7.13,;9.55,-7.91,;6.89,-4.81,;6.9,-3.27,;5.55,-5.57,;5.55,-4.04,;14.88,-7.91,;14.88,-9.45,;16.22,-10.22,;16.22,-11.76,;17.47,-12.66,;17,-14.13,;15.46,-14.13,;14.98,-12.67,;14.56,-15.38,;13.03,-15.22,;15.19,-16.79,;16.21,-7.14,;17.55,-7.9,;18.88,-7.13,;18.87,-5.58,;17.54,-4.82,;16.21,-5.59,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Activity at human recombinant S1P5 receptor expressed in CHO cells assessed as increase in calcium release by FLIPR assay |
Bioorg Med Chem Lett 17: 491-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.014
BindingDB Entry DOI: 10.7270/Q2028SCS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50250637

(CHEMBL4087932)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2C)c(OC)n1 |t:14| Show InChI InChI=1S/C27H34N2O4/c1-5-6-22-9-7-20(26(28-22)32-4)16-33-25-12-11-23-17(2)19(8-10-24(23)18(25)3)13-29-14-21(15-29)27(30)31/h7,9,11-12,21H,5-6,8,10,13-16H2,1-4H3,(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559619

(CHEMBL4798181)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CCC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(11.56,-5.73,;11.56,-7.27,;10.23,-8.04,;10.23,-9.59,;8.9,-10.35,;7.56,-9.58,;7.57,-8.04,;6.25,-7.27,;4.91,-8.03,;4.9,-9.57,;6.24,-10.35,;3.58,-7.25,;3.58,-5.71,;2.24,-8.02,;2.23,-6.48,;11.56,-10.36,;11.57,-11.9,;12.9,-12.66,;13.07,-14.19,;14.58,-14.51,;15.34,-13.17,;14.31,-12.03,;16.87,-13.01,;17.78,-14.25,;17.5,-11.6,;12.9,-9.58,;14.23,-10.35,;15.57,-9.57,;15.56,-8.02,;14.22,-7.26,;12.9,-8.03,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50428142

(CHEMBL2336071)Show SMILES CCc1cc(ccc1CN1CC(C1)C(O)=O)C(\C)=N\OCc1ccc(C2CCCCC2)c(c1)C(F)(F)F Show InChI InChI=1S/C29H35F3N2O3/c1-3-21-14-23(10-11-24(21)15-34-16-25(17-34)28(35)36)19(2)33-37-18-20-9-12-26(22-7-5-4-6-8-22)27(13-20)29(30,31)32/h9-14,22,25H,3-8,15-18H2,1-2H3,(H,35,36)/b33-19+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor expressed in CHO cells by [35S]GTPgammaS binding assay |
Bioorg Med Chem Lett 23: 6377-89 (2013)
Article DOI: 10.1016/j.bmcl.2013.09.058
BindingDB Entry DOI: 10.7270/Q2FR00KV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50250614

(CHEMBL4095920)Show SMILES CCCc1ccc(COc2ccc3C(C)=C(CN4CC(C4)C(O)=O)CCc3c2C)c(OC)c1 |t:14| Show InChI InChI=1S/C28H35NO4/c1-5-6-20-7-8-22(27(13-20)32-4)17-33-26-12-11-24-18(2)21(9-10-25(24)19(26)3)14-29-15-23(16-29)28(30)31/h7-8,11-13,23H,5-6,9-10,14-17H2,1-4H3,(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a |
Ono Pharmaceutical Co., Ltd
Curated by ChEMBL
| Assay Description
Displacement of [33P]-S1P from human S1P5 receptor expressed in CHO-K1 cells after 60 mins by scintillation counting method |
J Med Chem 60: 9508-9530 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00785
BindingDB Entry DOI: 10.7270/Q2J968SK |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559604
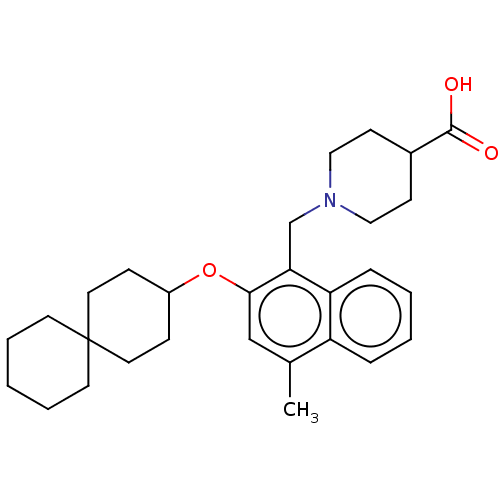
(CHEMBL4757160)Show SMILES Cc1cc(OC2CCC3(CCCCC3)CC2)c(CN2CCC(CC2)C(O)=O)c2ccccc12 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50532552
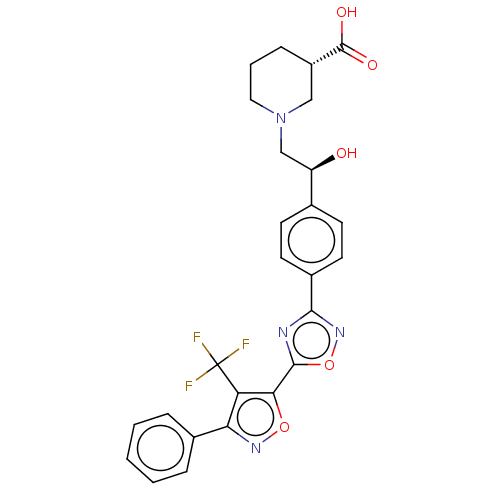
(CHEMBL4448752)Show SMILES O[C@H](CN1CCC[C@@H](C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C26H23F3N4O5/c27-26(28,29)20-21(16-5-2-1-3-6-16)31-37-22(20)24-30-23(32-38-24)17-10-8-15(9-11-17)19(34)14-33-12-4-7-18(13-33)25(35)36/h1-3,5-6,8-11,18-19,34H,4,7,12-14H2,(H,35,36)/t18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor (unknown origin) measured after 45 mins by [35S]GTP-gammaS binding assay |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50532552

(CHEMBL4448752)Show SMILES O[C@H](CN1CCC[C@@H](C1)C(O)=O)c1ccc(cc1)-c1noc(n1)-c1onc(c1C(F)(F)F)-c1ccccc1 |r| Show InChI InChI=1S/C26H23F3N4O5/c27-26(28,29)20-21(16-5-2-1-3-6-16)31-37-22(20)24-30-23(32-38-24)17-10-8-15(9-11-17)19(34)14-33-12-4-7-18(13-33)25(35)36/h1-3,5-6,8-11,18-19,34H,4,7,12-14H2,(H,35,36)/t18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor (unknown origin) measured after 45 mins by [35S]GTP-gammaS binding assay |
J Med Chem 59: 6248-64 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00373
BindingDB Entry DOI: 10.7270/Q2FJ2M8Z |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559625
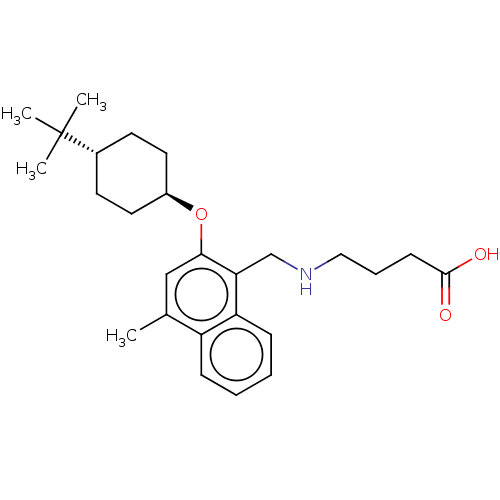
(CHEMBL4778095)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CNCCCC(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(11.95,-5.46,;11.95,-7,;10.62,-7.77,;10.62,-9.31,;9.29,-10.08,;7.95,-9.31,;7.96,-7.76,;6.64,-6.99,;5.3,-7.76,;5.29,-9.3,;6.63,-10.08,;3.97,-6.98,;3.97,-5.44,;2.63,-7.74,;2.62,-6.21,;11.95,-10.08,;11.96,-11.62,;13.29,-12.39,;13.3,-13.93,;14.63,-14.7,;14.64,-16.24,;15.97,-17.01,;15.97,-18.55,;17.3,-16.23,;13.29,-9.31,;14.62,-10.07,;15.96,-9.3,;15.95,-7.75,;14.61,-6.99,;13.29,-7.76,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559629
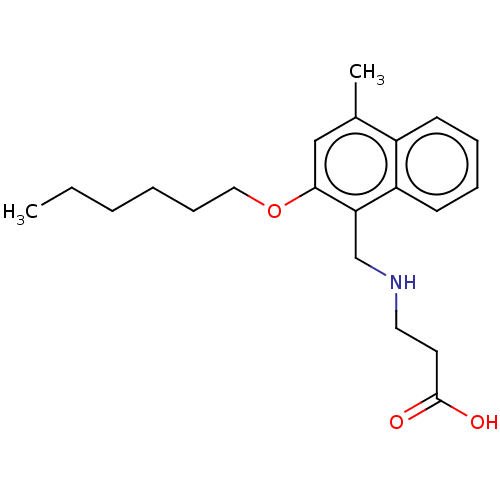
(CHEMBL4751484) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50314402
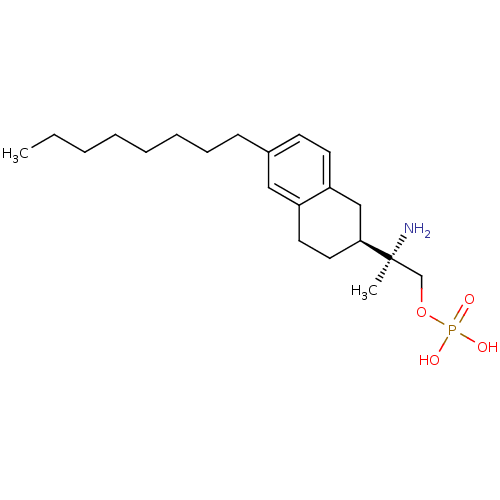
((R)-2-amino-2-((S)-6-octyl-1,2,3,4-tetrahydronapht...)Show SMILES CCCCCCCCc1ccc2C[C@H](CCc2c1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C21H36NO4P/c1-3-4-5-6-7-8-9-17-10-11-19-15-20(13-12-18(19)14-17)21(2,22)16-26-27(23,24)25/h10-11,14,20H,3-9,12-13,15-16,22H2,1-2H3,(H2,23,24,25)/t20-,21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.49 | n/a | n/a | n/a | n/a |
BiogenIdec Inc.
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor assessed as effect on calcium mobilization by Gq dependent whole cell assay |
Bioorg Med Chem Lett 20: 2264-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.006
BindingDB Entry DOI: 10.7270/Q2JS9QJS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a |
University of Virginia
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Sphingosine 1-phosphate receptor 5 expressed in HEK293T cells was determined using [gamma-35S]-GTP as radioli... |
Bioorg Med Chem Lett 14: 4903-6 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.030
BindingDB Entry DOI: 10.7270/Q26T0NCJ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50158348

((2S,3R,4E)-2-amino-3-hydroxyoctadec-4-en-1-yl dihy...)Show SMILES CCCCCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O |r| Show InChI InChI=1S/C18H38NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(20)17(19)16-24-25(21,22)23/h14-15,17-18,20H,2-13,16,19H2,1H3,(H2,21,22,23)/b15-14+/t17-,18+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5R expressed in HEK293T cells assessed as [35S]GTPgammaS binding after 30 mins by scintillation counting |
ACS Med Chem Lett 4: 942-7 (2013)
Article DOI: 10.1021/ml400194r
BindingDB Entry DOI: 10.7270/Q2RR2269 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM321333
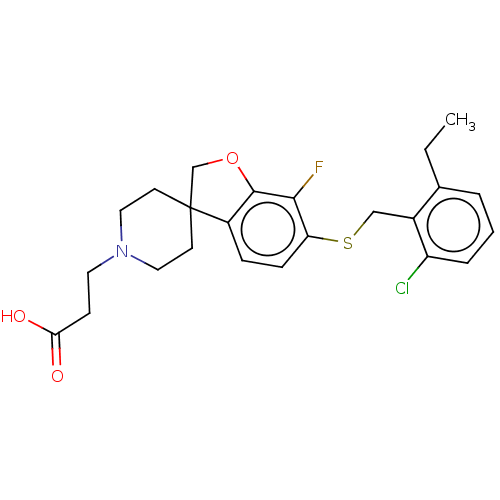
(US10179791, Compound 296 | US10807991, Compound 29...)Show SMILES CCc1cccc(Cl)c1CSc1ccc2c(OCC22CCN(CCC(O)=O)CC2)c1F Show InChI InChI=1S/C24H27ClFNO3S/c1-2-16-4-3-5-19(25)17(16)14-31-20-7-6-18-23(22(20)26)30-15-24(18)9-12-27(13-10-24)11-8-21(28)29/h3-7H,2,8-15H2,1H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
AbbVie B.V.; AbbVie, Inc.
US Patent
| Assay Description
The CHO-human-S1P5-Aeqorin assay was bought from Euroscreen, Brussels (Euroscreen, Technical dossier, Human Lysophospholid S1P5 (Edg8) receptor, DNA ... |
US Patent US10807991 (2020)
BindingDB Entry DOI: 10.7270/Q2X92FC5 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50475574
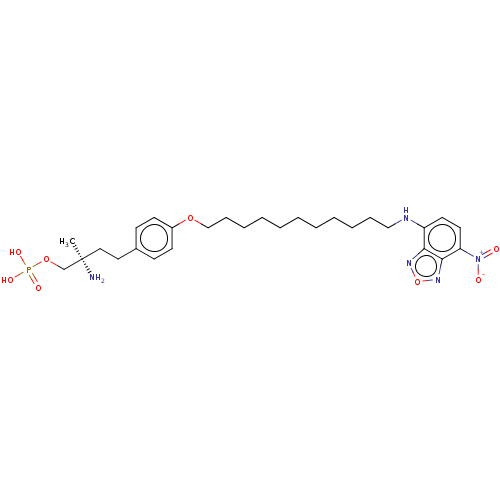
(CHEMBL371758)Show SMILES C[C@@](N)(CCc1ccc(OCCCCCCCCCCCNc2ccc([N+]([O-])=O)c3nonc23)cc1)COP(O)(O)=O Show InChI InChI=1S/C28H42N5O8P/c1-28(29,21-40-42(36,37)38)18-17-22-11-13-23(14-12-22)39-20-10-8-6-4-2-3-5-7-9-19-30-24-15-16-25(33(34)35)27-26(24)31-41-32-27/h11-16,30H,2-10,17-21,29H2,1H3,(H2,36,37,38)/t28-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding potency at human S1P5 receptor by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 16: 84-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.038
BindingDB Entry DOI: 10.7270/Q29C715H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50495090
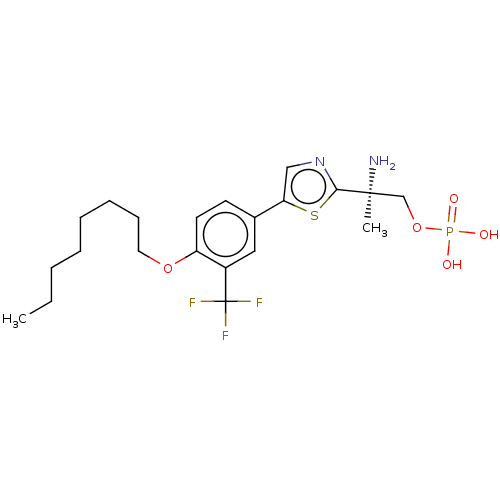
(CHEMBL3102903)Show SMILES CCCCCCCCOc1ccc(cc1C(F)(F)F)-c1cnc(s1)[C@@](C)(N)COP(O)(O)=O |r| Show InChI InChI=1S/C21H30F3N2O5PS/c1-3-4-5-6-7-8-11-30-17-10-9-15(12-16(17)21(22,23)24)18-13-26-19(33-18)20(2,25)14-31-32(27,28)29/h9-10,12-13H,3-8,11,14,25H2,1-2H3,(H2,27,28,29)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5R expressed in HEK293T cells assessed as [35S]GTPgammaS binding after 30 mins by scintillation counting |
ACS Med Chem Lett 4: 942-7 (2013)
Article DOI: 10.1021/ml400194r
BindingDB Entry DOI: 10.7270/Q2RR2269 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50475577

(CHEMBL382739)Show SMILES CCCCCCCOc1ccc(CC[C@@](C)(N)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C18H32NO5P/c1-3-4-5-6-7-14-23-17-10-8-16(9-11-17)12-13-18(2,19)15-24-25(20,21)22/h8-11H,3-7,12-15,19H2,1-2H3,(H2,20,21,22)/t18-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding potency at human S1P5 receptor by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 16: 84-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.038
BindingDB Entry DOI: 10.7270/Q29C715H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50475576
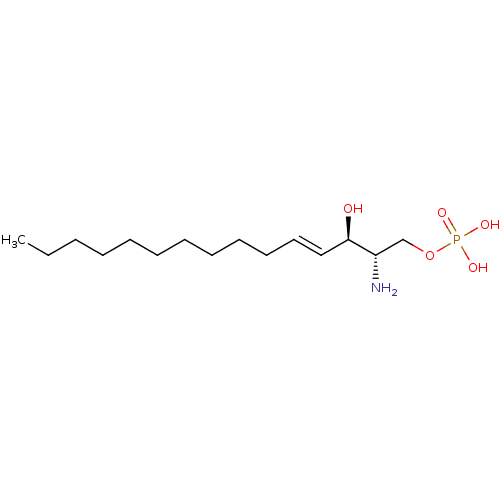
(CHEMBL199791)Show SMILES CCCCCCCCCC\C=C\[C@@H](O)[C@@H](N)COP(O)(O)=O Show InChI InChI=1S/C15H32NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-15(17)14(16)13-21-22(18,19)20/h11-12,14-15,17H,2-10,13,16H2,1H3,(H2,18,19,20)/b12-11+/t14-,15+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding potency at human S1P5 receptor by [35S]GTP-gamma-S binding assay |
Bioorg Med Chem Lett 16: 84-7 (2006)
Article DOI: 10.1016/j.bmcl.2005.09.038
BindingDB Entry DOI: 10.7270/Q29C715H |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM321333

(US10179791, Compound 296 | US10807991, Compound 29...)Show SMILES CCc1cccc(Cl)c1CSc1ccc2c(OCC22CCN(CCC(O)=O)CC2)c1F Show InChI InChI=1S/C24H27ClFNO3S/c1-2-16-4-3-5-19(25)17(16)14-31-20-7-6-18-23(22(20)26)30-15-24(18)9-12-27(13-10-24)11-8-21(28)29/h3-7H,2,8-15H2,1H3,(H,28,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
AbbVie B.V.; AbbVie, Inc.
US Patent
| Assay Description
The CHO-human-S1P5-Aeqorin assay was bought from Euroscreen, Brussels (Euroscreen, Technical dossier, Human Lysophospholid S1P5 (Edg8) receptor, DNA ... |
US Patent US10179791 (2019)
BindingDB Entry DOI: 10.7270/Q28K7C5D |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50313499
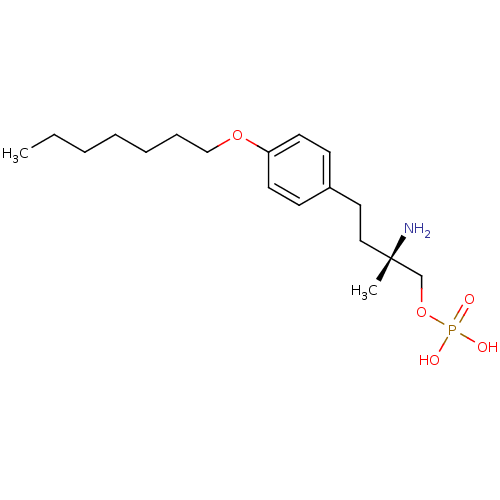
((S)-2-amino-4-(4-(heptyloxy)phenyl)-2-methylbutyl ...)Show SMILES CCCCCCCOc1ccc(CC[C@](C)(N)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C18H32NO5P/c1-3-4-5-6-7-14-23-17-10-8-16(9-11-17)12-13-18(2,19)15-24-25(20,21)22/h8-11H,3-7,12-15,19H2,1-2H3,(H2,20,21,22)/t18-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Agonist activity at human S1P5 receptor expressed in CHO cells assessed as induction of [S35]GTPgammaS binding |
Bioorg Med Chem Lett 20: 1485-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.118
BindingDB Entry DOI: 10.7270/Q2WD40QR |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50559621

(CHEMBL4796939)Show SMILES Cc1cc(O[C@H]2CC[C@@H](CC2)C(C)(C)C)c(CN2CC(C2)C(O)=O)c2ccccc12 |r,wU:8.11,wD:5.4,(11.68,-4.8,;11.69,-6.34,;10.36,-7.11,;10.36,-8.65,;9.02,-9.42,;7.69,-8.65,;7.7,-7.1,;6.37,-6.33,;5.03,-7.09,;5.03,-8.64,;6.36,-9.42,;3.7,-6.32,;3.71,-4.78,;2.36,-7.08,;2.36,-5.55,;11.69,-9.42,;11.69,-10.96,;13.03,-11.73,;13.44,-13.21,;14.92,-12.81,;14.52,-11.32,;16.26,-13.58,;16.26,-15.12,;17.59,-12.8,;13.02,-8.65,;14.36,-9.41,;15.69,-8.64,;15.68,-7.09,;14.35,-6.33,;13.02,-7.1,)| | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at recombinant human S1P5 receptor expressed in Chem-1 cells assessed as EC80 S1P-induced calcium flux measured for 180 secs by F... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00631
BindingDB Entry DOI: 10.7270/Q2D79G4R |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM195899
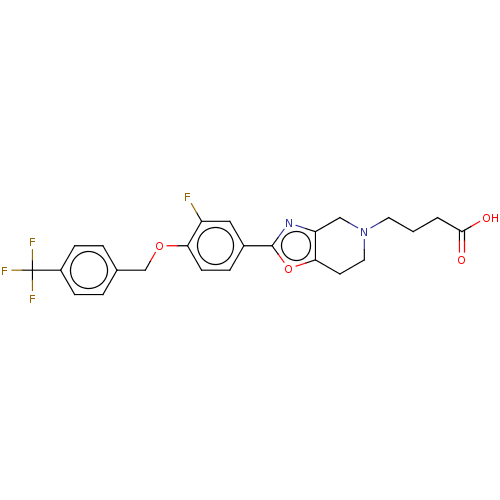
(US9670220, 85)Show SMILES OC(=O)CCCN1CCc2oc(nc2C1)-c1ccc(OCc2ccc(cc2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C24H22F4N2O4/c25-18-12-16(5-8-20(18)33-14-15-3-6-17(7-4-15)24(26,27)28)23-29-19-13-30(10-1-2-22(31)32)11-9-21(19)34-23/h3-8,12H,1-2,9-11,13-14H2,(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
Abbvie B.V.; AbbiVie Inc.
US Patent
| Assay Description
The CHO-human-S1P5-Aeqorin assay was bought from Euroscreen, Brussels (Euroscreen, Technical dossier, Human Lysophospholid S1P5 (Edg8) receptor, DNA ... |
US Patent US9670220 (2017)
BindingDB Entry DOI: 10.7270/Q2QJ7FHD |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM321316
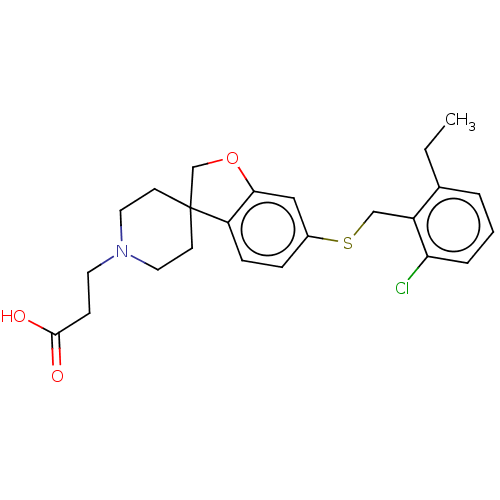
(US10179791, Compound 140 | US10807991, Compound 14...)Show SMILES CCc1cccc(Cl)c1CSc1ccc2c(OCC22CCN(CCC(O)=O)CC2)c1 Show InChI InChI=1S/C24H28ClNO3S/c1-2-17-4-3-5-21(25)19(17)15-30-18-6-7-20-22(14-18)29-16-24(20)9-12-26(13-10-24)11-8-23(27)28/h3-7,14H,2,8-13,15-16H2,1H3,(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Agonist activity at human S1P5 receptor expressed in CHO cells assessed as increase in calcium flux by aequorin-derived luminescence assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2017.12.018
BindingDB Entry DOI: 10.7270/Q2N87DCT |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM23164

(CHEMBL190006 | FTY720-phosphate, (R)-2 | [(2R)-2-a...)Show SMILES CCCCCCCCc1ccc(CC[C@@](N)(CO)COP(O)(O)=O)cc1 |r| Show InChI InChI=1S/C19H34NO5P/c1-2-3-4-5-6-7-8-17-9-11-18(12-10-17)13-14-19(20,15-21)16-25-26(22,23)24/h9-12,21H,2-8,13-16,20H2,1H3,(H2,22,23,24)/t19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Activity at human recombinant S1P5 receptor expressed in CHO cells assessed as increase in calcium release by FLIPR assay |
Bioorg Med Chem Lett 17: 491-4 (2007)
Article DOI: 10.1016/j.bmcl.2006.10.014
BindingDB Entry DOI: 10.7270/Q2028SCS |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM50186936

(3-(4-(5-(3-cyano-4-(1,1,1-trifluoropropan-2-yloxy)...)Show SMILES CC(Oc1ccc(cc1C#N)-c1nc(no1)-c1ccc(CCC(O)=O)cc1C)C(F)(F)F Show InChI InChI=1S/C22H18F3N3O4/c1-12-9-14(4-8-19(29)30)3-6-17(12)20-27-21(32-28-20)15-5-7-18(16(10-15)11-26)31-13(2)22(23,24)25/h3,5-7,9-10,13H,4,8H2,1-2H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Agonist activity at S1P5 receptor expressed in CHO cells measured as S1P-induced [35S]GTPgammaS uptake |
Bioorg Med Chem Lett 16: 3679-83 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.084
BindingDB Entry DOI: 10.7270/Q2J67GJ8 |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 5
(Homo sapiens (Human)) | BDBM321316

(US10179791, Compound 140 | US10807991, Compound 14...)Show SMILES CCc1cccc(Cl)c1CSc1ccc2c(OCC22CCN(CCC(O)=O)CC2)c1 Show InChI InChI=1S/C24H28ClNO3S/c1-2-17-4-3-5-21(25)19(17)15-30-18-6-7-20-22(14-18)29-16-24(20)9-12-26(13-10-24)11-8-23(27)28/h3-7,14H,2,8-13,15-16H2,1H3,(H,27,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a |
AbbVie B.V.; AbbVie, Inc.
US Patent
| Assay Description
The CHO-human-S1P5-Aeqorin assay was bought from Euroscreen, Brussels (Euroscreen, Technical dossier, Human Lysophospholid S1P5 (Edg8) receptor, DNA ... |
US Patent US10807991 (2020)
BindingDB Entry DOI: 10.7270/Q2X92FC5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data