 Found 139 hits of kd for UniProtKB: P35367
Found 139 hits of kd for UniProtKB: P35367 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50341447
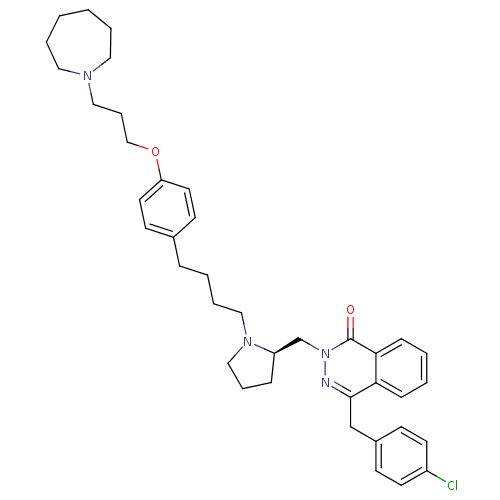
(4-[(4-Chlorophenyl)methyl]-2-({(2R)-1-[4-(4-{[3-(h...)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C39H49ClN4O2/c40-33-19-15-32(16-20-33)29-38-36-13-3-4-14-37(36)39(45)44(41-38)30-34-12-9-27-43(34)26-8-5-11-31-17-21-35(22-18-31)46-28-10-25-42-23-6-1-2-7-24-42/h3-4,13-22,34H,1-2,5-12,23-30H2/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418269
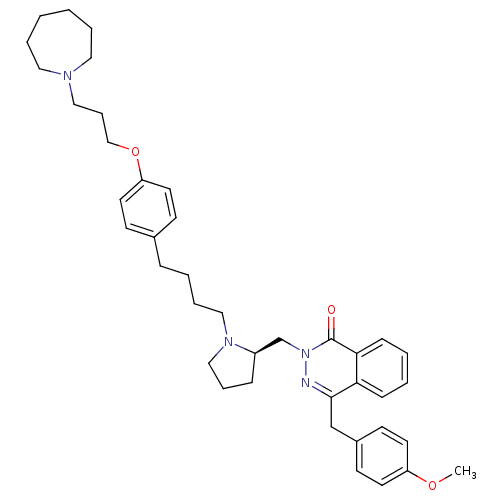
(CHEMBL1767165)Show SMILES COc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C40H52N4O3/c1-46-35-20-18-33(19-21-35)30-39-37-14-4-5-15-38(37)40(45)44(41-39)31-34-13-10-28-43(34)27-9-6-12-32-16-22-36(23-17-32)47-29-11-26-42-24-7-2-3-8-25-42/h4-5,14-23,34H,2-3,6-13,24-31H2,1H3/t34-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418270
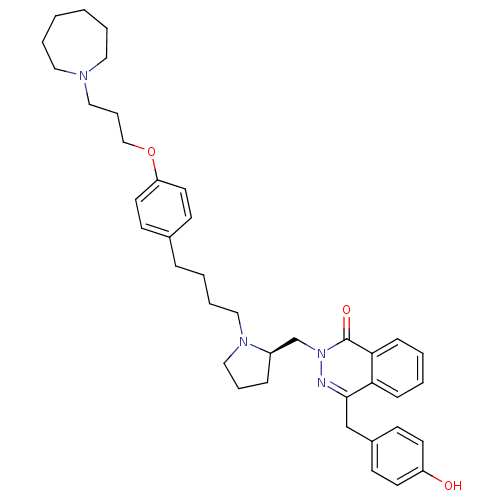
(CHEMBL1767166)Show SMILES Oc1ccc(Cc2nn(C[C@H]3CCCN3CCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C39H50N4O3/c44-34-19-15-32(16-20-34)29-38-36-13-3-4-14-37(36)39(45)43(40-38)30-33-12-9-27-42(33)26-8-5-11-31-17-21-35(22-18-31)46-28-10-25-41-23-6-1-2-7-24-41/h3-4,13-22,33,44H,1-2,5-12,23-30H2/t33-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50341448

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show SMILES CN1CCCC(CC1)n1nc(Cc2ccc(Cl)cc2)c2ccccc2c1=O Show InChI InChI=1S/C22H24ClN3O/c1-25-13-4-5-18(12-14-25)26-22(27)20-7-3-2-6-19(20)21(24-26)15-16-8-10-17(23)11-9-16/h2-3,6-11,18H,4-5,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50418271
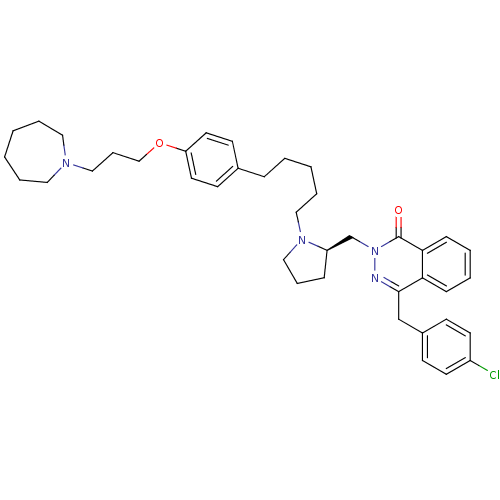
(CHEMBL1767167)Show SMILES Clc1ccc(Cc2nn(C[C@H]3CCCN3CCCCCc3ccc(OCCCN4CCCCCC4)cc3)c(=O)c3ccccc23)cc1 |r| Show InChI InChI=1S/C40H51ClN4O2/c41-34-20-16-33(17-21-34)30-39-37-14-5-6-15-38(37)40(46)45(42-39)31-35-13-10-28-44(35)27-9-3-4-12-32-18-22-36(23-19-32)47-29-11-26-43-24-7-1-2-8-25-43/h5-6,14-23,35H,1-4,7-13,24-31H2/t35-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre
Curated by ChEMBL
| Assay Description
Antagonist activity at human recombinant histamine H1 receptor expressed in intact CHO cells assessed as inhibition of histamine-induced cellular cal... |
J Med Chem 54: 2183-95 (2011)
Article DOI: 10.1021/jm1013874
BindingDB Entry DOI: 10.7270/Q2GQ6Z2Z |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50175517
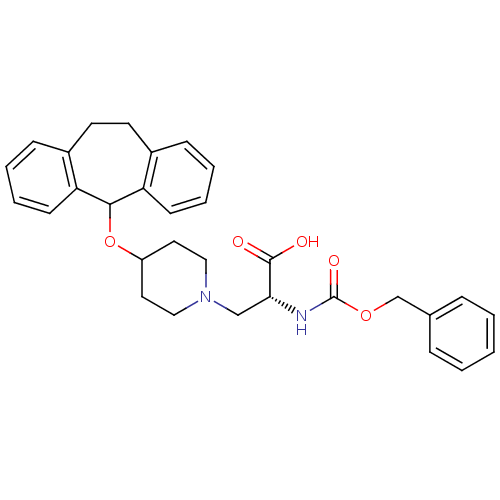
((R)-2-Benzyloxycarbonylamino-3-[4-(10,11-dihydro-5...)Show SMILES OC(=O)[C@@H](CN1CCC(CC1)OC1c2ccccc2CCc2ccccc12)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C31H34N2O5/c34-30(35)28(32-31(36)37-21-22-8-2-1-3-9-22)20-33-18-16-25(17-19-33)38-29-26-12-6-4-10-23(26)14-15-24-11-5-7-13-27(24)29/h1-13,25,28-29H,14-21H2,(H,32,36)(H,34,35)/t28-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50017721

(1-Methyl-4-(5H-dibenzo(a,d)cycloheptenylidene)pipe...)Show SMILES [#6]-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccccc2-[#6]=[#6]-c2ccccc-12 |c:16| Show InChI InChI=1S/C21H21N/c1-22-14-12-18(13-15-22)21-19-8-4-2-6-16(19)10-11-17-7-3-5-9-20(17)21/h2-11H,12-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a |
Organon Laboratories
Curated by ChEMBL
| Assay Description
Dissociation constant against histamine H1 receptor |
J Med Chem 48: 6523-43 (2005)
Article DOI: 10.1021/jm058225d
BindingDB Entry DOI: 10.7270/Q2SF2WZ9 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM35254

(2-methyl-4-(4-methylpiperazin-1-yl)-10H-thieno[2,3...)Show InChI InChI=1S/C17H20N4S/c1-12-11-13-16(21-9-7-20(2)8-10-21)18-14-5-3-4-6-15(14)19-17(13)22-12/h3-6,11,19H,7-10H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 0.0870 | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity to human histamine H1 receptor |
J Med Chem 54: 26-53 (2011)
Article DOI: 10.1021/jm100064d
BindingDB Entry DOI: 10.7270/Q2VQ33RV |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM22876
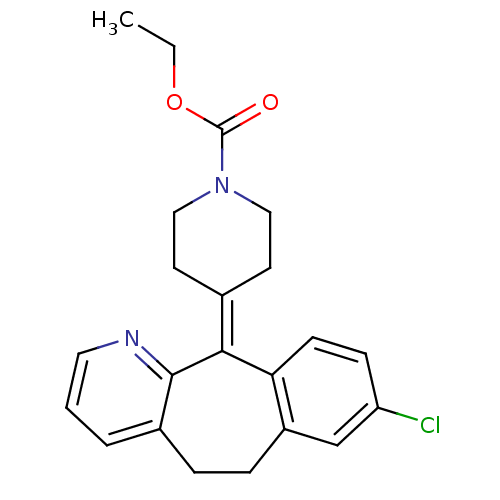
(CHEMBL998 | Claritin | Loratadine | Sch 29851 | US...)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545588
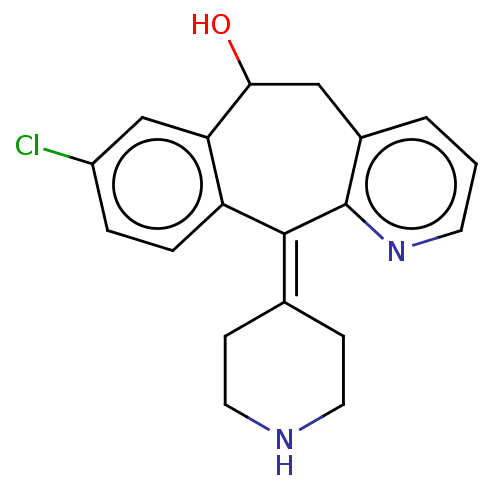
(CHEMBL4647890)Show SMILES [#8]-[#6]-1-[#6]-c2cccnc2\[#6](=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)-c2ccc(Cl)cc-12 Show InChI InChI=1S/C19H19ClN2O/c20-14-3-4-15-16(11-14)17(23)10-13-2-1-7-22-19(13)18(15)12-5-8-21-9-6-12/h1-4,7,11,17,21,23H,5-6,8-10H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545589
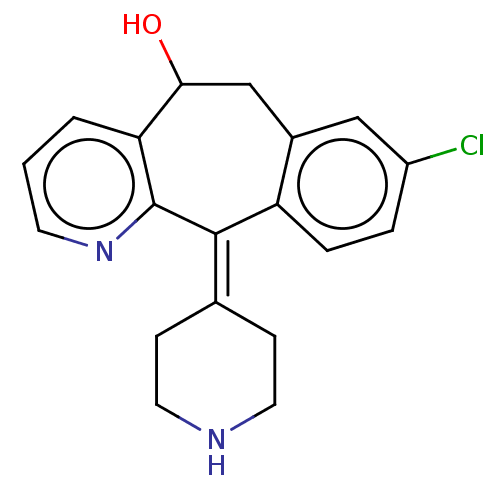
(CHEMBL4647038)Show SMILES [#8]-[#6]-1-[#6]-c2cc(Cl)ccc2\[#6](=[#6]-2\[#6]-[#6]-[#7]-[#6]-[#6]-2)-c2ncccc-12 Show InChI InChI=1S/C19H19ClN2O/c20-14-3-4-15-13(10-14)11-17(23)16-2-1-7-22-19(16)18(15)12-5-8-21-9-6-12/h1-4,7,10,17,21,23H,5-6,8-9,11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50073179
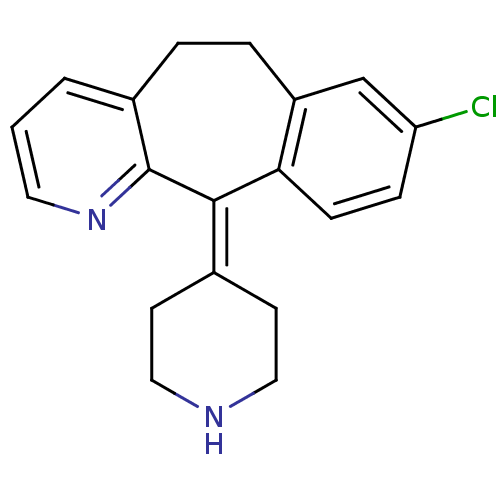
(8-Chloro-11-piperidin-4-ylidene-6,11-dihydro-5H-be...)Show SMILES Clc1ccc2c(-[#6]-[#6]-c3cccnc3\[#6]-2=[#6]-2/[#6]-[#6]-[#7]-[#6]-[#6]-2)c1 Show InChI InChI=1S/C19H19ClN2/c20-16-5-6-17-15(12-16)4-3-14-2-1-9-22-19(14)18(17)13-7-10-21-11-8-13/h1-2,5-6,9,12,21H,3-4,7-8,10-11H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545590
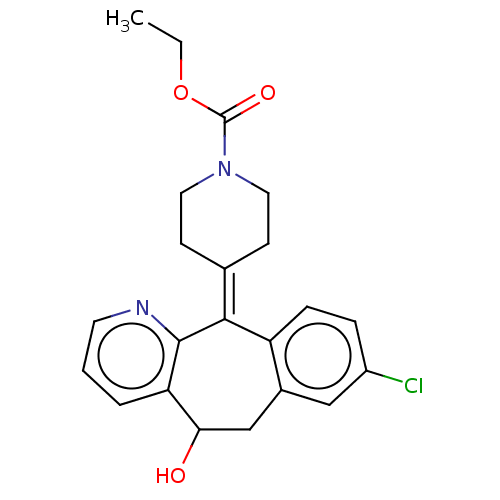
(CHEMBL4645629)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6](-[#8])-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O3/c1-2-28-22(27)25-10-7-14(8-11-25)20-17-6-5-16(23)12-15(17)13-19(26)18-4-3-9-24-21(18)20/h3-6,9,12,19,26H,2,7-8,10-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 454 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545591
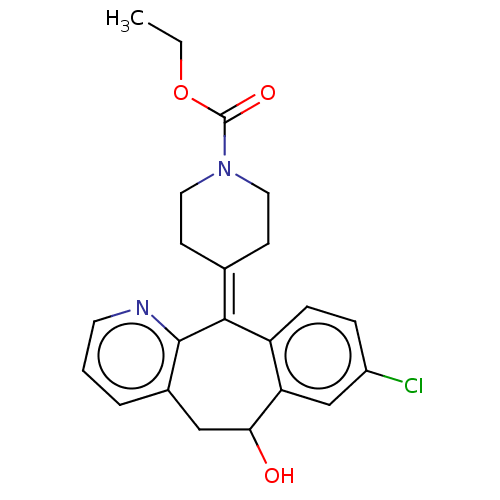
(CHEMBL4639563)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6](-[#8])-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O3/c1-2-28-22(27)25-10-7-14(8-11-25)20-17-6-5-16(23)13-18(17)19(26)12-15-4-3-9-24-21(15)20/h3-6,9,13,19,26H,2,7-8,10-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 257 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545592
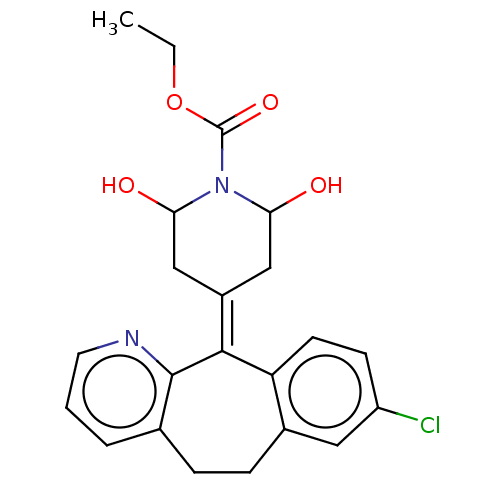
(CHEMBL4637657)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6](-[#8])-[#6]\[#6](-[#6]-[#6]-1-[#8])=[#6]-1/c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O4/c1-2-29-22(28)25-18(26)11-15(12-19(25)27)20-17-8-7-16(23)10-14(17)6-5-13-4-3-9-24-21(13)20/h3-4,7-10,18-19,26-27H,2,5-6,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545593
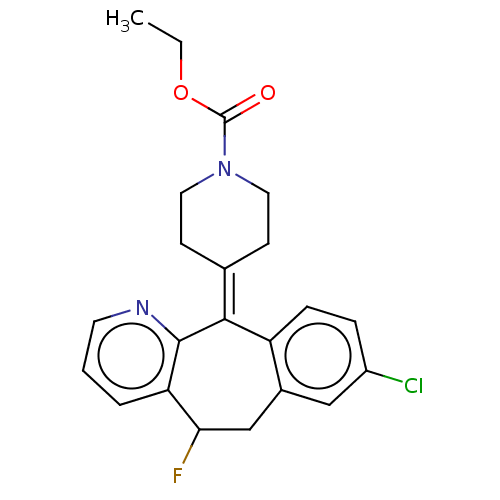
(CHEMBL4638183)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6](F)-c2cccnc-12 Show InChI InChI=1S/C22H22ClFN2O2/c1-2-28-22(27)26-10-7-14(8-11-26)20-17-6-5-16(23)12-15(17)13-19(24)18-4-3-9-25-21(18)20/h3-6,9,12,19H,2,7-8,10-11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 227 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545594
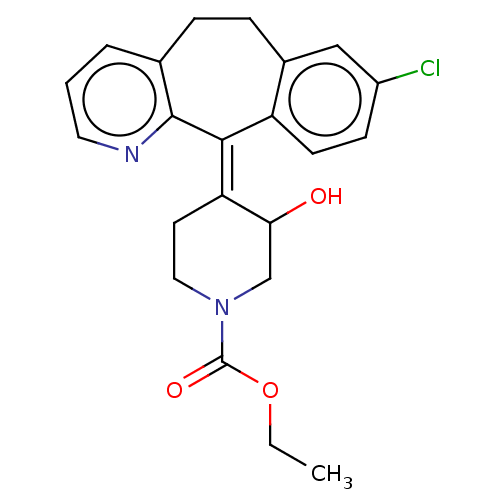
(CHEMBL4649344)Show SMILES CCOC(=O)N1CC\C(C(O)C1)=C1\c2ccc(Cl)cc2CCc2cccnc12 Show InChI InChI=1S/C22H23ClN2O3/c1-2-28-22(27)25-11-9-18(19(26)13-25)20-17-8-7-16(23)12-15(17)6-5-14-4-3-10-24-21(14)20/h3-4,7-8,10,12,19,26H,2,5-6,9,11,13H2,1H3/b20-18+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 423 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545595
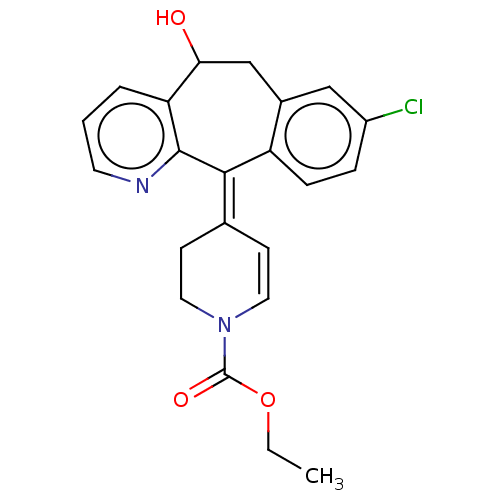
(CHEMBL4633258)Show SMILES CCOC(=O)N1CC\C(C=C1)=C1\c2ccc(Cl)cc2CC(O)c2cccnc12 |c:9| Show InChI InChI=1S/C22H21ClN2O3/c1-2-28-22(27)25-10-7-14(8-11-25)20-17-6-5-16(23)12-15(17)13-19(26)18-4-3-9-24-21(18)20/h3-7,9-10,12,19,26H,2,8,11,13H2,1H3/b20-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50031236

(CHEMBL3357023)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccc(Cl)cc2-[#6]-[#6]-c2cc(-[#8])cnc-12 Show InChI InChI=1S/C22H23ClN2O3/c1-2-28-22(27)25-9-7-14(8-10-25)20-19-6-5-17(23)11-15(19)3-4-16-12-18(26)13-24-21(16)20/h5-6,11-13,26H,2-4,7-10H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545596

(CHEMBL4633111)Show SMILES CCOC(=O)N1CC\C(C=C1)=C1/c2ccc(Cl)cc2CCc2cc(O)cnc12 |c:9| Show InChI InChI=1S/C22H21ClN2O3/c1-2-28-22(27)25-9-7-14(8-10-25)20-19-6-5-17(23)11-15(19)3-4-16-12-18(26)13-24-21(16)20/h5-7,9,11-13,26H,2-4,8,10H2,1H3/b20-14+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545597

(CHEMBL4643460)Show SMILES CCOC(=O)N1CC\C(C=C1)=C1\c2ccc(Cl)cc2CCc2cc(O)cnc12 |c:9| Show InChI InChI=1S/C22H21ClN2O3/c1-2-28-22(27)25-9-7-14(8-10-25)20-19-6-5-17(23)11-15(19)3-4-16-12-18(26)13-24-21(16)20/h5-7,9,11-13,26H,2-4,8,10H2,1H3/b20-14- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545598
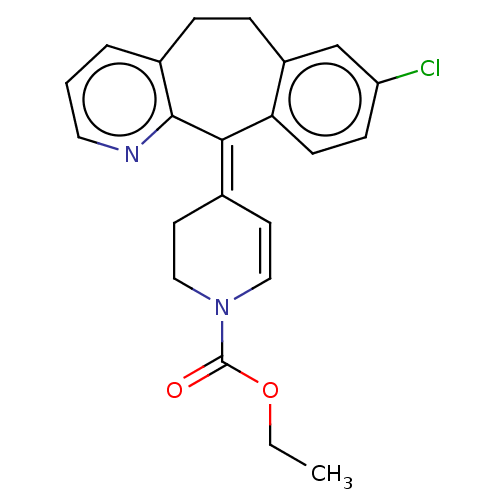
(CHEMBL4649552)Show SMILES CCOC(=O)N1CC\C(C=C1)=C1\c2ccc(Cl)cc2CCc2cccnc12 |c:9| Show InChI InChI=1S/C22H21ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-9,11-12,14H,2,5-6,10,13H2,1H3/b20-15- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545599
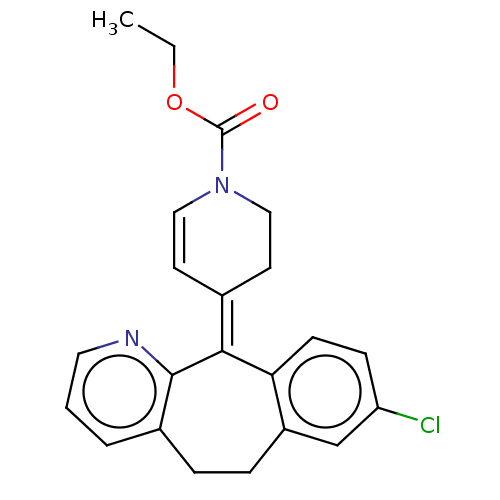
(CHEMBL4642056)Show SMILES CCOC(=O)N1CC\C(C=C1)=C1/c2ccc(Cl)cc2CCc2cccnc12 |c:9| Show InChI InChI=1S/C22H21ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-4,7-9,11-12,14H,2,5-6,10,13H2,1H3/b20-15+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 454 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545600
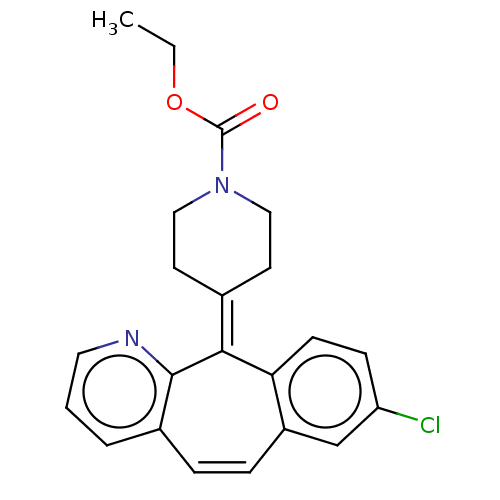
(CHEMBL4641273)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]=[#6]-c2cccnc-12 |c:21| Show InChI InChI=1S/C22H21ClN2O2/c1-2-27-22(26)25-12-9-15(10-13-25)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-24-21(16)20/h3-8,11,14H,2,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545601
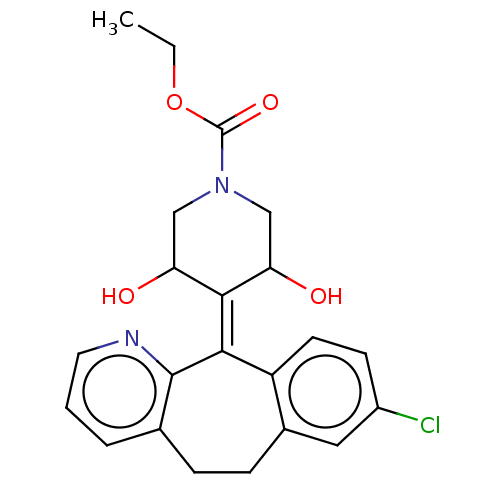
(CHEMBL4646840)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6](-[#8])\[#6](-[#6](-[#8])-[#6]-1)=[#6]-1/c2ccc(Cl)cc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H23ClN2O4/c1-2-29-22(28)25-11-17(26)20(18(27)12-25)19-16-8-7-15(23)10-14(16)6-5-13-4-3-9-24-21(13)19/h3-4,7-10,17-18,26-27H,2,5-6,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545602
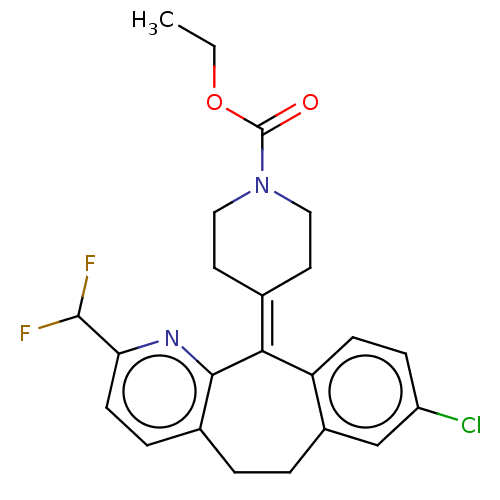
(CHEMBL4634510)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2ccc(nc-12)-[#6](F)F Show InChI InChI=1S/C23H23ClF2N2O2/c1-2-30-23(29)28-11-9-14(10-12-28)20-18-7-6-17(24)13-16(18)4-3-15-5-8-19(22(25)26)27-21(15)20/h5-8,13,22H,2-4,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545603
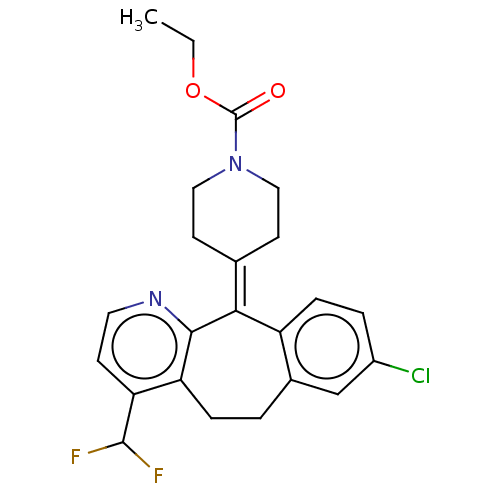
(CHEMBL4633975)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2c(ccnc-12)-[#6](F)F Show InChI InChI=1S/C23H23ClF2N2O2/c1-2-30-23(29)28-11-8-14(9-12-28)20-17-6-4-16(24)13-15(17)3-5-18-19(22(25)26)7-10-27-21(18)20/h4,6-7,10,13,22H,2-3,5,8-9,11-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 271 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50031281
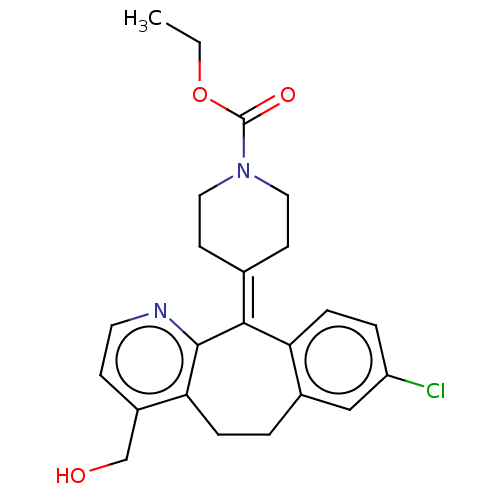
(CHEMBL3357022)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1/c2ccc(Cl)cc2-[#6]-[#6]-c2c(-[#6]-[#8])ccnc-12 Show InChI InChI=1S/C23H25ClN2O3/c1-2-29-23(28)26-11-8-15(9-12-26)21-19-6-4-18(24)13-16(19)3-5-20-17(14-27)7-10-25-22(20)21/h4,6-7,10,13,27H,2-3,5,8-9,11-12,14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 199 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545604
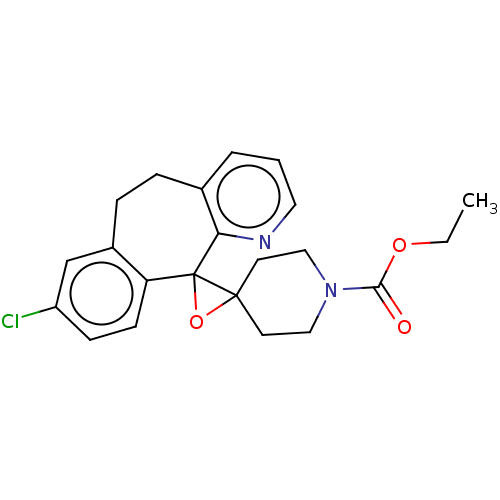
(CHEMBL4634809)Show SMILES CCOC(=O)N1CCC2(CC1)OC21c2ccc(Cl)cc2CCc2cccnc12 Show InChI InChI=1S/C22H23ClN2O3/c1-2-27-20(26)25-12-9-21(10-13-25)22(28-21)18-8-7-17(23)14-16(18)6-5-15-4-3-11-24-19(15)22/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545605
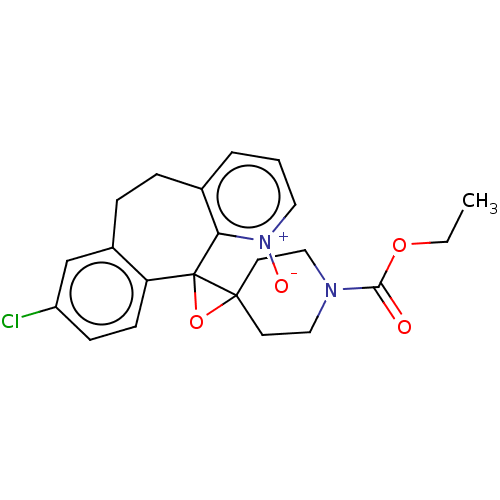
(CHEMBL4648718)Show SMILES CCOC(=O)N1CCC2(CC1)OC21c2ccc(Cl)cc2CCc2ccc[n+]([O-])c12 Show InChI InChI=1S/C22H23ClN2O4/c1-2-28-20(26)24-12-9-21(10-13-24)22(29-21)18-8-7-17(23)14-16(18)6-5-15-4-3-11-25(27)19(15)22/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545606
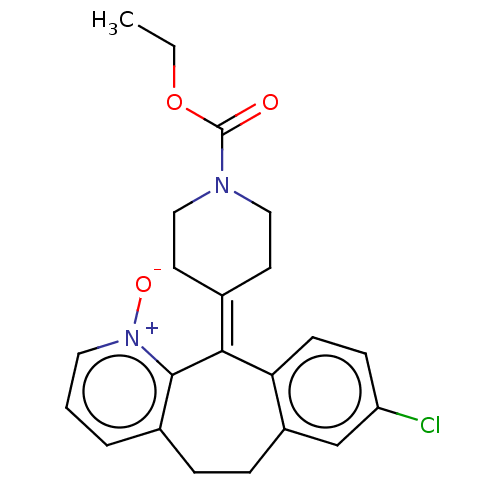
(CHEMBL4649329)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2ccc[n+](-[#8-])c-12 Show InChI InChI=1S/C22H23ClN2O3/c1-2-28-22(26)24-12-9-15(10-13-24)20-19-8-7-18(23)14-17(19)6-5-16-4-3-11-25(27)21(16)20/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545607
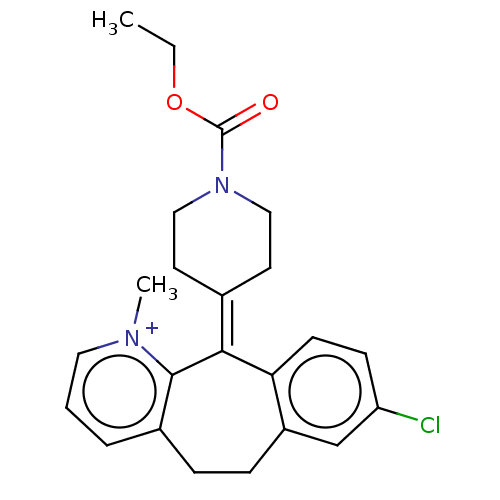
(CHEMBL4632879)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccc(Cl)cc2-[#6]-[#6]-c2ccc[n+](-[#6])c-12 Show InChI InChI=1S/C23H26ClN2O2/c1-3-28-23(27)26-13-10-16(11-14-26)21-20-9-8-19(24)15-18(20)7-6-17-5-4-12-25(2)22(17)21/h4-5,8-9,12,15H,3,6-7,10-11,13-14H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545608

(CHEMBL4632720)Show SMILES CCOC(=O)N1CCC(=O)C2(CC1)c1ccc(Cl)cc1CCc1cccnc21 Show InChI InChI=1S/C22H23ClN2O3/c1-2-28-21(27)25-12-9-19(26)22(10-13-25)18-8-7-17(23)14-16(18)6-5-15-4-3-11-24-20(15)22/h3-4,7-8,11,14H,2,5-6,9-10,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50545609
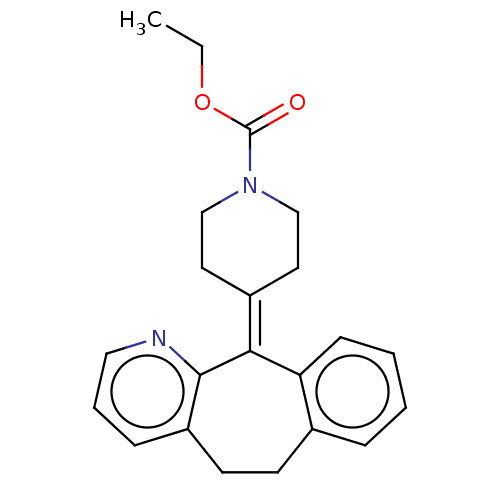
(CHEMBL4642460)Show SMILES [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]-1\c2ccccc2-[#6]-[#6]-c2cccnc-12 Show InChI InChI=1S/C22H24N2O2/c1-2-26-22(25)24-14-11-17(12-15-24)20-19-8-4-3-6-16(19)9-10-18-7-5-13-23-21(18)20/h3-8,13H,2,9-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 206 | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Binding affinity to human H1 histamine receptor expressed in CHO cells after 1 hr by calcium 5 dye-based FLIPR assay |
J Med Chem 63: 7268-7292 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00483
BindingDB Entry DOI: 10.7270/Q2KP85RR |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50601586

(CHEMBL5194860)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(CCCc1ccccc1)CNC(=O)\N=C(/N)NCC1CCc2nc(N)sc2C1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50601587
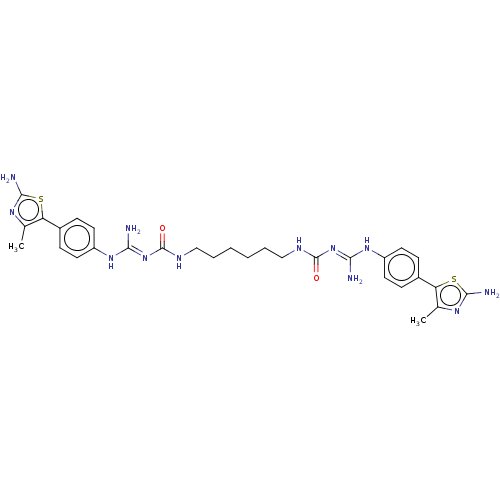
(CHEMBL5172675)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1nc(N)sc1-c1ccc(N\C(N)=N\C(=O)NCCCCCCNC(=O)\N=C(/N)Nc2ccc(cc2)-c2sc(N)nc2C)cc1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50601588
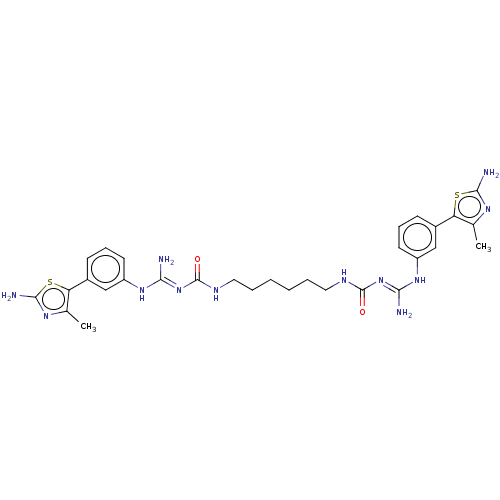
(CHEMBL5174278)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.Cc1nc(N)sc1-c1cccc(N\C(N)=N\C(=O)NCCCCCCNC(=O)\N=C(/N)Nc2cccc(c2)-c2sc(N)nc2C)c1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50601589

(CHEMBL5176229)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50601590
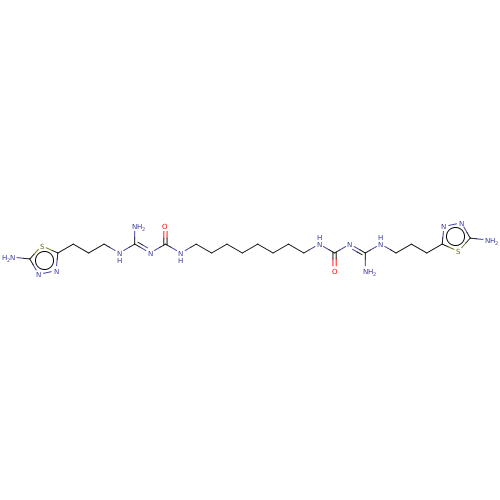
(CHEMBL5173079)Show SMILES OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.N\C(NCCCc1nnc(N)s1)=N/C(=O)NCCCCCCCCNC(=O)\N=C(/N)NCCCc1nnc(N)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50601591
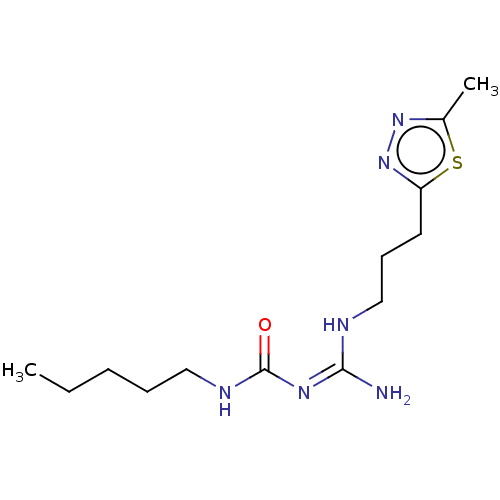
(CHEMBL5187758)Show SMILES OC(=O)C(F)(F)F.CCCCCNC(=O)\N=C(\N)NCCCc1nnc(C)s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00692
BindingDB Entry DOI: 10.7270/Q2MP57CT |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606399

(CHEMBL5198557)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:65,67,t:62| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606400
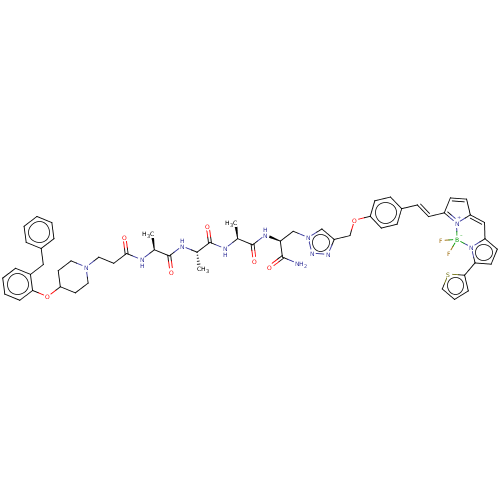
(CHEMBL5201153)Show SMILES C[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:59,61,t:56| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606401

(CHEMBL5208512)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CCC(O)=O)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:68,70,t:65| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606402

(CHEMBL5183487)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)NCCC(=O)N[C@@H](CCCn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:72,74,t:69| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606403
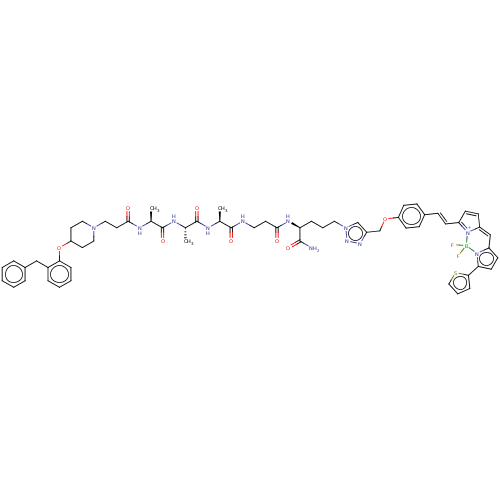
(CHEMBL5171227)Show SMILES C[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)NCCC(=O)N[C@@H](CCCn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:66,68,t:63| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
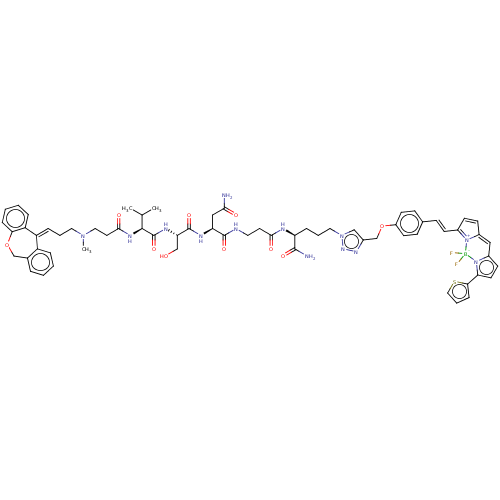
(Homo sapiens (Human)) | BDBM50606404
(CHEMBL5194541)Show SMILES CC(C)[C@H](NC(=O)CCN(C)CC\C=C1/c2ccccc2COc2ccccc12)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)NCCC(=O)N[C@@H](CCCn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |c:72,74,t:69| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606405

(CHEMBL5180096)Show SMILES C[C@H](NC(=O)CCN(C)CC\C=C1/c2ccccc2COc2ccccc12)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)NCCC(=O)N[C@@H](CCCn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:66,68,t:63| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606399

(CHEMBL5198557)Show SMILES CC(C)[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](CO)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](Cn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:65,67,t:62| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |
Histamine H1 receptor
(Homo sapiens (Human)) | BDBM50606400

(CHEMBL5201153)Show SMILES C[C@H](NC(=O)CCN1CCC(CC1)Oc1ccccc1Cc1ccccc1)C(=O)N[C@@H](C)C(=O)N[C@@H](C)C(=O)N[C@@H](Cn1cc(COc2ccc(\C=C\C3=[N+]4C(C=C3)=Cc3ccc(-c5cccs5)n3[B-]4(F)F)cc2)nn1)C(N)=O |r,c:59,61,t:56| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00125
BindingDB Entry DOI: 10.7270/Q29W0KK1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data