

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268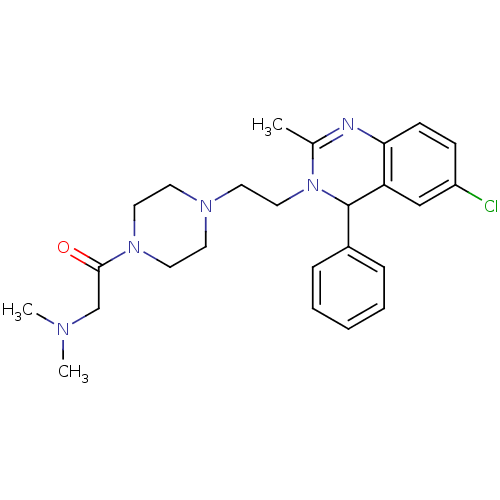 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354299 (CHEMBL1836378) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257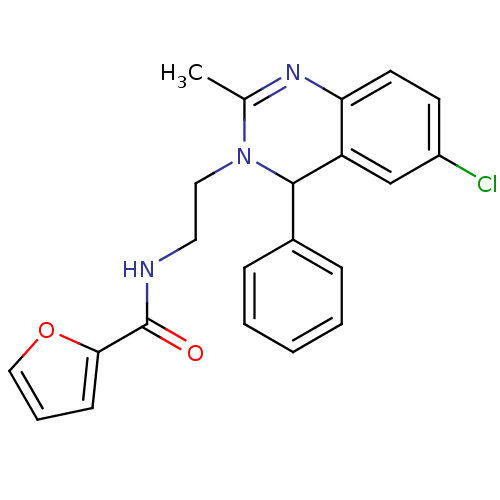 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50096500 (CHEMBL86281 | N,N'-Bis-(3-phenyl-propyl)-N,N'-bis-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354279 (CHEMBL1836603) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354268 (CHEMBL1836570) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50354257 (CHEMBL1836559) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Mixed type inhibition of recombinant trypanothione reductase from Trypanosoma brucei brucei S427 by Lineweaver burk method | J Med Chem 54: 6514-30 (2011) Article DOI: 10.1021/jm200312v BindingDB Entry DOI: 10.7270/Q2G73F4P | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485931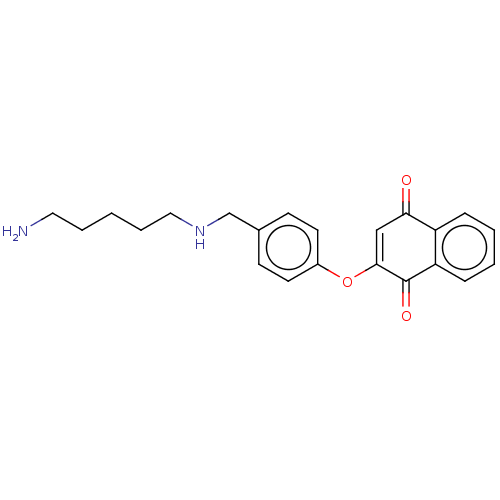 (CHEMBL2178999) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-inhibitor c... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50096496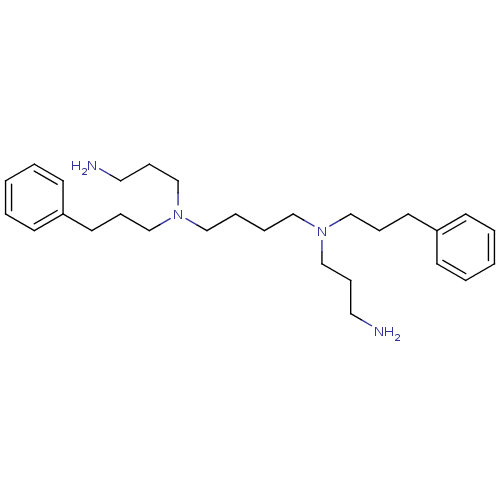 (CHEMBL62048 | N,N'-Bis-(3-amino-propyl)-N,N'-bis-(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485930 (CHEMBL2179000) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-inhibitor c... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485930 (CHEMBL2179000) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Noncompetitive inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide by Lineweaver-Burk plot | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485930 (CHEMBL2179000) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-substrate-i... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50485931 (CHEMBL2178999) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bologna Curated by ChEMBL | Assay Description Mixed type inhibition of Trypanosoma brucei TR using varying levels of trypanothione disulfide assessed as inhibition constant for enzyme-substrate-i... | J Med Chem 55: 10490-500 (2012) Article DOI: 10.1021/jm301112z BindingDB Entry DOI: 10.7270/Q2BC42D7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50462235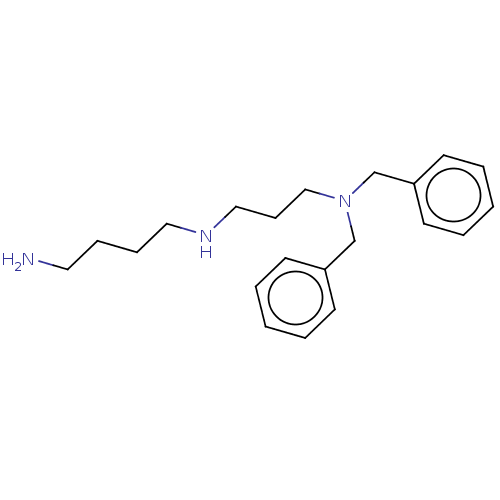 (CHEMBL4244566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay Curated by ChEMBL | Assay Description Mixed-type inhibition of Trypanosoma brucei trypanothione reductase assessed as enzyme-inhibitor complex using varying levels of TS2 as substrate in ... | Eur J Med Chem 150: 655-666 (2018) Article DOI: 10.1016/j.ejmech.2018.02.087 BindingDB Entry DOI: 10.7270/Q22N54ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50096498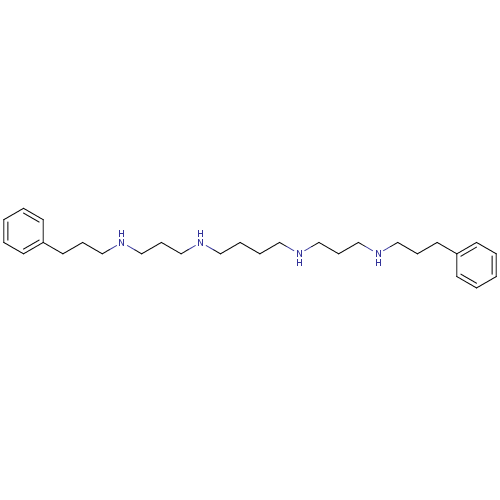 (CHEMBL86096 | N,N'-Bis-[3-(3-phenyl-propylamino)-p...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50009371 (1, 12-DB-3-4-3 | CHEMBL81614 | N,N'-Bis-(3-benzyla...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50462235 (CHEMBL4244566) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Saclay Curated by ChEMBL | Assay Description Mixed-type inhibition of Trypanosoma brucei trypanothione reductase assessed as enzyme-inhibitor-substrate complex using varying levels of TS2 as sub... | Eur J Med Chem 150: 655-666 (2018) Article DOI: 10.1016/j.ejmech.2018.02.087 BindingDB Entry DOI: 10.7270/Q22N54ZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trypanothione reductase (Trypanosoma brucei brucei) | BDBM50615382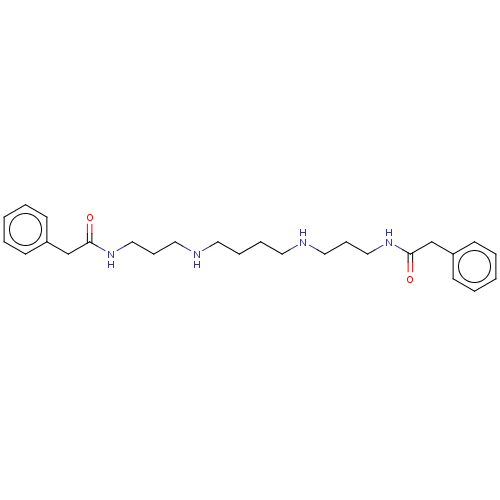 (CHEMBL211029) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | UniChem | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||