 Found 229 hits of kd for UniProtKB: P22303
Found 229 hits of kd for UniProtKB: P22303 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91725

(Carbacylamidophosphate, 2a)Show InChI InChI=1S/C7H5BrCl2NO2P/c8-6-3-1-5(2-4-6)7(12)11-14(9,10)13/h1-4H,(H,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70E+4 | 2.30E+5 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91729

(Carbacylamidophosphate, 4a)Show InChI InChI=1S/C8H8Cl2NO2P/c1-6-2-4-7(5-3-6)8(12)11-14(9,10)13/h2-5H,1H3,(H,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+5 | 5.54E+5 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91727

(Carbacylamidophosphate, 3a)Show InChI InChI=1S/C7H6Cl2NO2P/c8-13(9,12)10-7(11)6-4-2-1-3-5-6/h1-5H,(H,10,11,12) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.85E+5 | 1.10E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91726
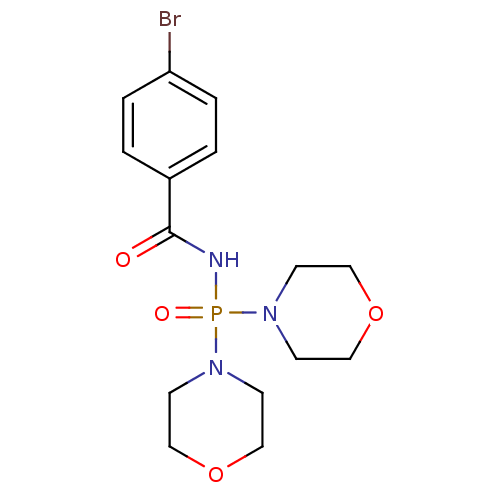
(Carbacylamidophosphate, 2b)Show InChI InChI=1S/C15H21BrN3O4P/c16-14-3-1-13(2-4-14)15(20)17-24(21,18-5-9-22-10-6-18)19-7-11-23-12-8-19/h1-4H,5-12H2,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20E+5 | 3.82E+3 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91724
(Carbacylamidophosphate, 1b)Show InChI InChI=1S/C15H21ClN3O4P/c16-14-3-1-13(2-4-14)15(20)17-24(21,18-5-9-22-10-6-18)19-7-11-23-12-8-19/h1-4H,5-12H2,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.32E+5 | 2.39E+5 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91730

(Carbacylamidophosphate, 4b)Show InChI InChI=1S/C16H24N3O4P/c1-14-2-4-15(5-3-14)16(20)17-24(21,18-6-10-22-11-7-18)19-8-12-23-13-9-19/h2-5H,6-13H2,1H3,(H,17,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.89E+6 | 4.26E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91728

(Carbacylamidophosphate, 3b)Show InChI InChI=1S/C15H22N3O4P/c19-15(14-4-2-1-3-5-14)16-23(20,17-6-10-21-11-7-17)18-8-12-22-13-9-18/h1-5H,6-13H2,(H,16,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.15E+6 | 2.95E+6 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM91723
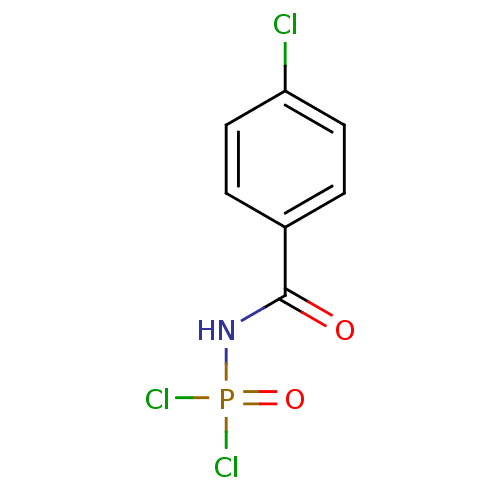
(Carbacylamidophosphate, 1a)Show InChI InChI=1S/C7H5Cl3NO2P/c8-6-3-1-5(2-4-6)7(12)11-14(9,10)13/h1-4H,(H,11,12,13) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 8.70E+7 | 2.06E+5 | n/a | n/a | n/a | 7.4 | 37 |
Tarbiat Modares University
| Assay Description
In-vitro and vivo using AChE and BuChE enzyme. |
J Enzyme Inhib Med Chem 24: 566-76 (2009)
Article DOI: 10.1080/14756360802316971
BindingDB Entry DOI: 10.7270/Q2VT1QPP |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005558
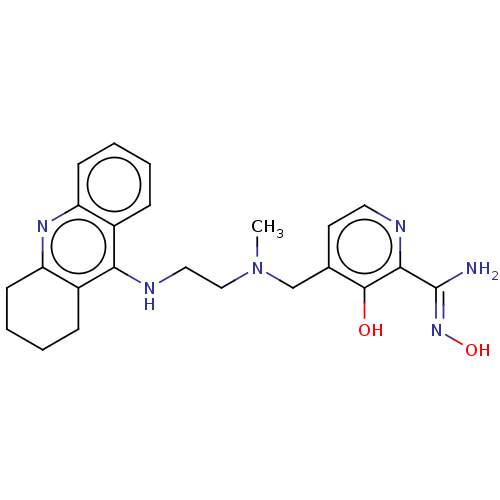
(CHEMBL138554 | CHEMBL3233028)Show SMILES CN(CCNc1c2CCCCc2nc2ccccc12)Cc1ccnc(\C(N)=N\O)c1O Show InChI InChI=1S/C23H28N6O2/c1-29(14-15-10-11-25-21(22(15)30)23(24)28-31)13-12-26-20-16-6-2-4-8-18(16)27-19-9-5-3-7-17(19)20/h2,4,6,8,10-11,30-31H,3,5,7,9,12-14H2,1H3,(H2,24,28)(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005562
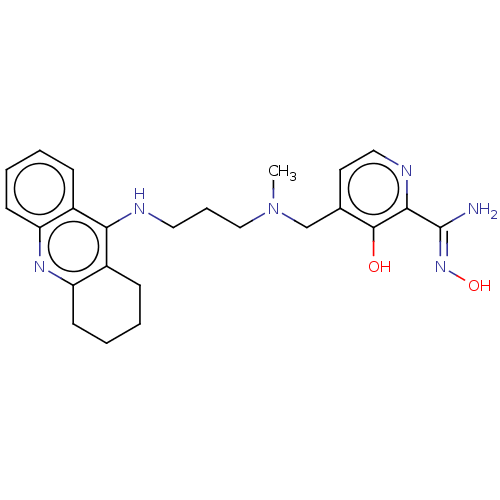
(CHEMBL138290 | CHEMBL3234586)Show SMILES CN(CCCNc1c2CCCCc2nc2ccccc12)Cc1ccnc(\C(N)=N\O)c1O Show InChI InChI=1S/C24H30N6O2/c1-30(15-16-11-13-27-22(23(16)31)24(25)29-32)14-6-12-26-21-17-7-2-4-9-19(17)28-20-10-5-3-8-18(20)21/h2,4,7,9,11,13,31-32H,3,5-6,8,10,12,14-15H2,1H3,(H2,25,29)(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005563
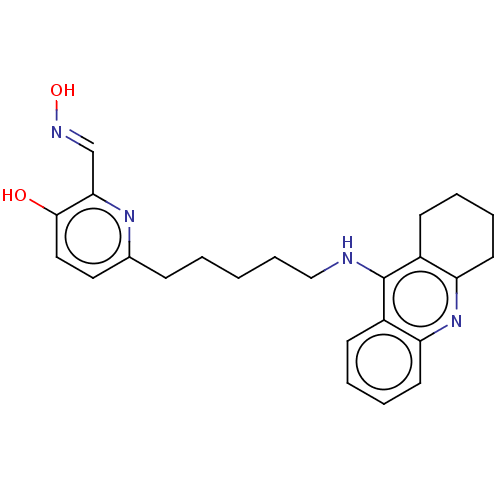
(CHEMBL3234587 | CHEMBL434456)Show SMILES O\N=C\c1nc(CCCCCNc2c3CCCCc3nc3ccccc23)ccc1O Show InChI InChI=1S/C24H28N4O2/c29-23-14-13-17(27-22(23)16-26-30)8-2-1-7-15-25-24-18-9-3-5-11-20(18)28-21-12-6-4-10-19(21)24/h3,5,9,11,13-14,16,29-30H,1-2,4,6-8,10,12,15H2,(H,25,28)/b26-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.10E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005566
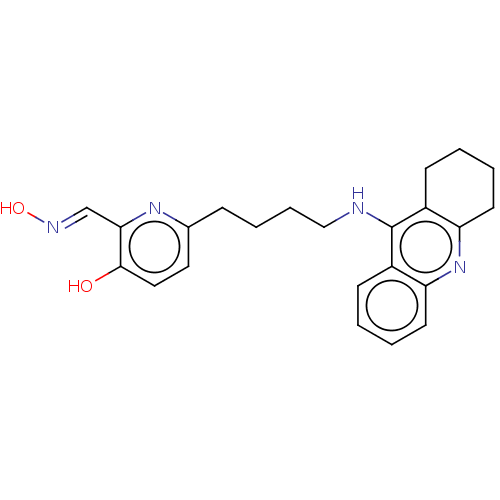
(CHEMBL139018 | CHEMBL3234588)Show InChI InChI=1S/C23H26N4O2/c28-22-13-12-16(26-21(22)15-25-29)7-5-6-14-24-23-17-8-1-3-10-19(17)27-20-11-4-2-9-18(20)23/h1,3,8,10,12-13,15,28-29H,2,4-7,9,11,14H2,(H,24,27)/b25-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.10E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50011780
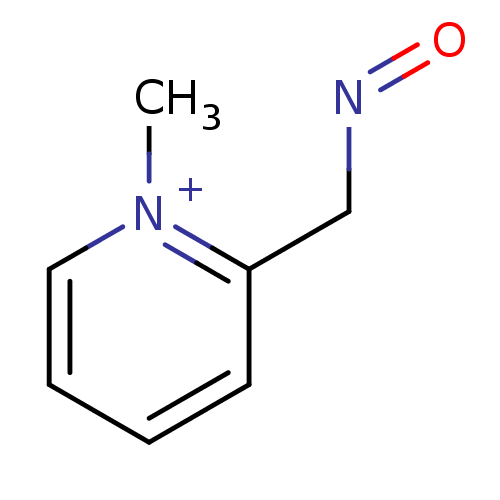
(2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...)Show InChI InChI=1S/C7H9N2O/c1-9-5-3-2-4-7(9)6-8-10/h2-5H,6H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.15E+5 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005568

(CHEMBL141735 | CHEMBL3234584)Show SMILES [Cl-].[Cl-].NC(=O)c1cc[n+](CCC[n+]2ccccc2\C=N\O)cc1 Show InChI InChI=1S/C15H16N4O2.2ClH/c16-15(20)13-5-10-18(11-6-13)7-3-9-19-8-2-1-4-14(19)12-17-21;;/h1-2,4-6,8,10-12H,3,7,9H2,(H-,16,20);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005571
(CHEMBL139138 | CHEMBL2181429)Show SMILES CS([O-])(=O)=O.CS([O-])(=O)=O.NC(=O)c1cc[n+](COC[n+]2ccc(\C=N\O)cc2\C=N\O)cc1 Show InChI InChI=1S/C15H15N5O4.2CH4O3S/c16-15(21)13-2-4-19(5-3-13)10-24-11-20-6-1-12(8-17-22)7-14(20)9-18-23;2*1-5(2,3)4/h1-9H,10-11H2,(H2-,16,21,22,23);2*1H3,(H,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005579
(CHEMBL139148 | Obidoxime)Show InChI InChI=1S/C14H14N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-10H,11-12H2/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of methylphosphonothioate VX-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005563

(CHEMBL3234587 | CHEMBL434456)Show SMILES O\N=C\c1nc(CCCCCNc2c3CCCCc3nc3ccccc23)ccc1O Show InChI InChI=1S/C24H28N4O2/c29-23-14-13-17(27-22(23)16-26-30)8-2-1-7-15-25-24-18-9-3-5-11-20(18)28-21-12-6-4-10-19(21)24/h3,5,9,11,13-14,16,29-30H,1-2,4,6-8,10,12,15H2,(H,25,28)/b26-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of tabun-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005566

(CHEMBL139018 | CHEMBL3234588)Show InChI InChI=1S/C23H26N4O2/c28-22-13-12-16(26-21(22)15-25-29)7-5-6-14-24-23-17-8-1-3-10-19(17)27-20-11-4-2-9-18(20)23/h1,3,8,10,12-13,15,28-29H,2,4-7,9,11,14H2,(H,24,27)/b25-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of tabun-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005571

(CHEMBL139138 | CHEMBL2181429)Show SMILES CS([O-])(=O)=O.CS([O-])(=O)=O.NC(=O)c1cc[n+](COC[n+]2ccc(\C=N\O)cc2\C=N\O)cc1 Show InChI InChI=1S/C15H15N5O4.2CH4O3S/c16-15(21)13-2-4-19(5-3-13)10-24-11-20-6-1-12(8-17-22)7-14(20)9-18-23;2*1-5(2,3)4/h1-9H,10-11H2,(H2-,16,21,22,23);2*1H3,(H,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of tabun-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005579

(CHEMBL139148 | Obidoxime)Show InChI InChI=1S/C14H14N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-10H,11-12H2/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 2.50E+5 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of tabun-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50041520
(1,3-PROPYLENE-BIS-N,N''-SYN-4-PYRIDINIUMALDOXIME |...)Show InChI InChI=1S/C15H18N4O2/c20-16-12-14-2-8-18(9-3-14)6-1-7-19-10-4-15(5-11-19)13-17-21/h2-5,8-11H,1,6-7,12-13H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of tabun-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005563

(CHEMBL3234587 | CHEMBL434456)Show SMILES O\N=C\c1nc(CCCCCNc2c3CCCCc3nc3ccccc23)ccc1O Show InChI InChI=1S/C24H28N4O2/c29-23-14-13-17(27-22(23)16-26-30)8-2-1-7-15-25-24-18-9-3-5-11-20(18)28-21-12-6-4-10-19(21)24/h3,5,9,11,13-14,16,29-30H,1-2,4,6-8,10,12,15H2,(H,25,28)/b26-16+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of paraoxon-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005566

(CHEMBL139018 | CHEMBL3234588)Show InChI InChI=1S/C23H26N4O2/c28-22-13-12-16(26-21(22)15-25-29)7-5-6-14-24-23-17-8-1-3-10-19(17)27-20-11-4-2-9-18(20)23/h1,3,8,10,12-13,15,28-29H,2,4-7,9,11,14H2,(H,24,27)/b25-15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of paraoxon-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005571

(CHEMBL139138 | CHEMBL2181429)Show SMILES CS([O-])(=O)=O.CS([O-])(=O)=O.NC(=O)c1cc[n+](COC[n+]2ccc(\C=N\O)cc2\C=N\O)cc1 Show InChI InChI=1S/C15H15N5O4.2CH4O3S/c16-15(21)13-2-4-19(5-3-13)10-24-11-20-6-1-12(8-17-22)7-14(20)9-18-23;2*1-5(2,3)4/h1-9H,10-11H2,(H2-,16,21,22,23);2*1H3,(H,2,3,4) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of paraoxon-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50005579

(CHEMBL139148 | Obidoxime)Show InChI InChI=1S/C14H14N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-10H,11-12H2/p+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of paraoxon-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50041520

(1,3-PROPYLENE-BIS-N,N''-SYN-4-PYRIDINIUMALDOXIME |...)Show InChI InChI=1S/C15H18N4O2/c20-16-12-14-2-8-18(9-3-14)6-1-7-19-10-4-15(5-11-19)13-17-21/h2-5,8-11H,1,6-7,12-13H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.78E+4 | n/a | n/a | n/a | n/a | n/a |
Universit£ de Strasbourg
Curated by ChEMBL
| Assay Description
Reactivation of paraoxon-inhibited recombinant human AChE measured up to 10 mins by Ellman's method |
Eur J Med Chem 78: 455-67 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.044
BindingDB Entry DOI: 10.7270/Q2RJ4M0X |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013122
(CHEMBL3261988)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C20H24N6O4.2BrH/c27-19(13-21-29)23-17-7-5-11-25(15-17)9-3-1-2-4-10-26-12-6-8-18(16-26)24-20(28)14-22-30;;/h5-8,11-16H,1-4,9-10H2,(H2-2,23,24,27,28,29,30);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.44E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013125
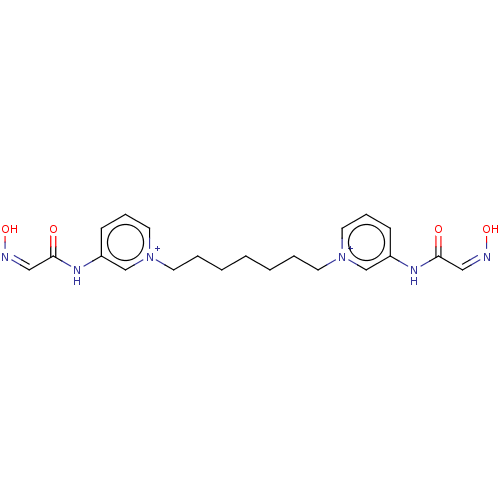
(CHEMBL3261989)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCCCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C21H26N6O4.2BrH/c28-20(14-22-30)24-18-8-6-12-26(16-18)10-4-2-1-3-5-11-27-13-7-9-19(17-27)25-21(29)15-23-31;;/h6-9,12-17H,1-5,10-11H2,(H2-2,24,25,28,29,30,31);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.43E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013126
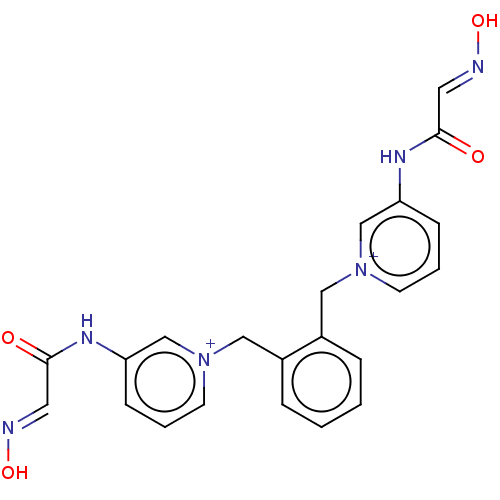
(CHEMBL3261990)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](Cc2ccccc2C[n+]2cccc(NC(=O)\C=N\O)c2)c1 Show InChI InChI=1S/C22H20N6O4.2BrH/c29-21(11-23-31)25-19-7-3-9-27(15-19)13-17-5-1-2-6-18(17)14-28-10-4-8-20(16-28)26-22(30)12-24-32;;/h1-12,15-16H,13-14H2,(H2-2,25,26,29,30,31,32);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013127
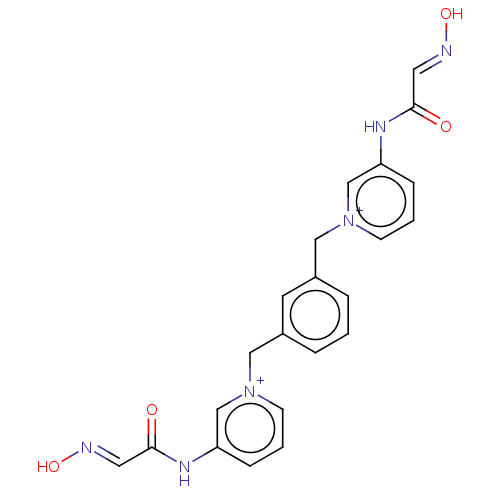
(CHEMBL3261991)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](Cc2cccc(C[n+]3cccc(NC(=O)\C=N\O)c3)c2)c1 Show InChI InChI=1S/C22H20N6O4.2BrH/c29-21(11-23-31)25-19-6-2-8-27(15-19)13-17-4-1-5-18(10-17)14-28-9-3-7-20(16-28)26-22(30)12-24-32;;/h1-12,15-16H,13-14H2,(H2-2,25,26,29,30,31,32);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.84E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013120
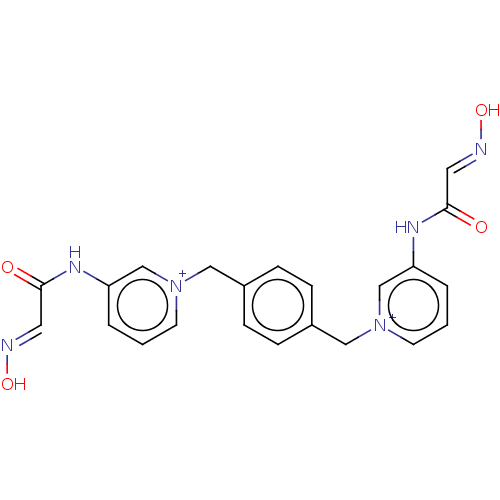
(CHEMBL3261992)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](Cc2ccc(C[n+]3cccc(NC(=O)\C=N\O)c3)cc2)c1 Show InChI InChI=1S/C22H20N6O4.2BrH/c29-21(11-23-31)25-19-3-1-9-27(15-19)13-17-5-7-18(8-6-17)14-28-10-2-4-20(16-28)26-22(30)12-24-32;;/h1-12,15-16H,13-14H2,(H2-2,25,26,29,30,31,32);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.55E+3 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50011780

(2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...)Show InChI InChI=1S/C7H9N2O/c1-9-5-3-2-4-7(9)6-8-10/h2-5H,6H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.57E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50333779

(1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...)Show InChI InChI=1S/C14H16N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-8H,9-12H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013129

(CHEMBL3261984)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](CC[n+]2cccc(NC(=O)\C=N\O)c2)c1 Show InChI InChI=1S/C16H16N6O4.2BrH/c23-15(9-17-25)19-13-3-1-5-21(11-13)7-8-22-6-2-4-14(12-22)20-16(24)10-18-26;;/h1-6,9-12H,7-8H2,(H2-2,19,20,23,24,25,26);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.87E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013130
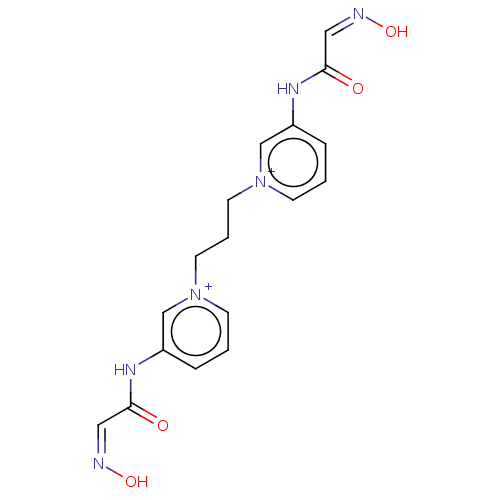
(CHEMBL3261985)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C17H18N6O4.2BrH/c24-16(10-18-26)20-14-4-1-6-22(12-14)8-3-9-23-7-2-5-15(13-23)21-17(25)11-19-27;;/h1-2,4-7,10-13H,3,8-9H2,(H2-2,20,21,24,25,26,27);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.89E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013132
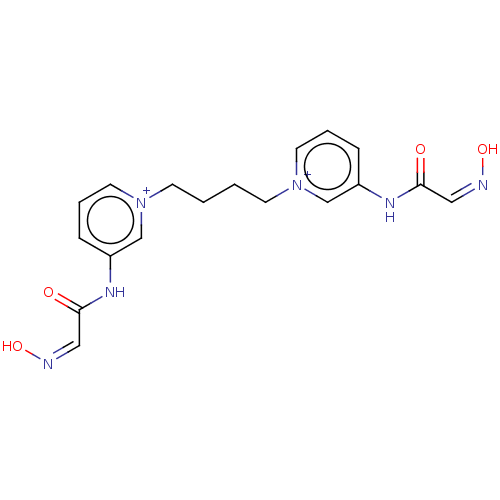
(CHEMBL3261986)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C18H20N6O4.2BrH/c25-17(11-19-27)21-15-5-3-9-23(13-15)7-1-2-8-24-10-4-6-16(14-24)22-18(26)12-20-28;;/h3-6,9-14H,1-2,7-8H2,(H2-2,21,22,25,26,27,28);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.61E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013133
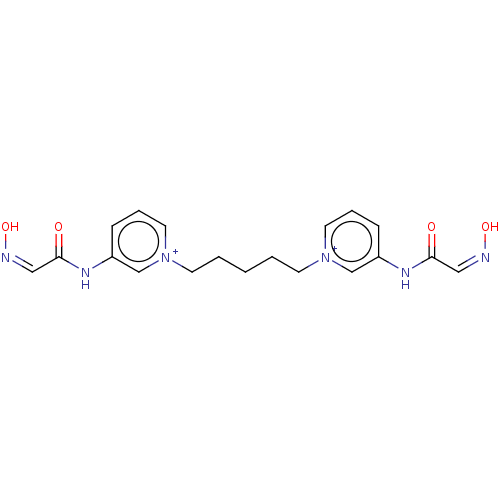
(CHEMBL3261987)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C19H22N6O4.2BrH/c26-18(12-20-28)22-16-6-4-10-24(14-16)8-2-1-3-9-25-11-5-7-17(15-25)23-19(27)13-21-29;;/h4-7,10-15H,1-3,8-9H2,(H2-2,22,23,26,27,28,29);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013122

(CHEMBL3261988)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C20H24N6O4.2BrH/c27-19(13-21-29)23-17-7-5-11-25(15-17)9-3-1-2-4-10-26-12-6-8-18(16-26)24-20(28)14-22-30;;/h5-8,11-16H,1-4,9-10H2,(H2-2,23,24,27,28,29,30);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013125

(CHEMBL3261989)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCCCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C21H26N6O4.2BrH/c28-20(14-22-30)24-18-8-6-12-26(16-18)10-4-2-1-3-5-11-27-13-7-9-19(17-27)25-21(29)15-23-31;;/h6-9,12-17H,1-5,10-11H2,(H2-2,24,25,28,29,30,31);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 6.58E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013126

(CHEMBL3261990)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](Cc2ccccc2C[n+]2cccc(NC(=O)\C=N\O)c2)c1 Show InChI InChI=1S/C22H20N6O4.2BrH/c29-21(11-23-31)25-19-7-3-9-27(15-19)13-17-5-1-2-6-18(17)14-28-10-4-8-20(16-28)26-22(30)12-24-32;;/h1-12,15-16H,13-14H2,(H2-2,25,26,29,30,31,32);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.26E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013127

(CHEMBL3261991)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](Cc2cccc(C[n+]3cccc(NC(=O)\C=N\O)c3)c2)c1 Show InChI InChI=1S/C22H20N6O4.2BrH/c29-21(11-23-31)25-19-6-2-8-27(15-19)13-17-4-1-5-18(10-17)14-28-9-3-7-20(16-28)26-22(30)12-24-32;;/h1-12,15-16H,13-14H2,(H2-2,25,26,29,30,31,32);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.49E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013120

(CHEMBL3261992)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](Cc2ccc(C[n+]3cccc(NC(=O)\C=N\O)c3)cc2)c1 Show InChI InChI=1S/C22H20N6O4.2BrH/c29-21(11-23-31)25-19-3-1-9-27(15-19)13-17-5-7-18(8-6-17)14-28-10-2-4-20(16-28)26-22(30)12-24-32;;/h1-12,15-16H,13-14H2,(H2-2,25,26,29,30,31,32);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50011780

(2-[(E)-(hydroxyimino)methyl]-1-methylpyridinium ch...)Show InChI InChI=1S/C7H9N2O/c1-9-5-3-2-4-7(9)6-8-10/h2-5H,6H2,1H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.14E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50333779

(1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxap...)Show InChI InChI=1S/C14H16N4O3/c19-15-9-13-1-5-17(6-2-13)11-21-12-18-7-3-14(4-8-18)10-16-20/h1-8H,9-12H2/q+2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to VX-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured up ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013129

(CHEMBL3261984)Show SMILES [Br-].[Br-].O\N=C\C(=O)Nc1ccc[n+](CC[n+]2cccc(NC(=O)\C=N\O)c2)c1 Show InChI InChI=1S/C16H16N6O4.2BrH/c23-15(9-17-25)19-13-3-1-5-21(11-13)7-8-22-6-2-4-14(12-22)20-16(24)10-18-26;;/h1-6,9-12H,7-8H2,(H2-2,19,20,23,24,25,26);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4.29E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013130

(CHEMBL3261985)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C17H18N6O4.2BrH/c24-16(10-18-26)20-14-4-1-6-22(12-14)8-3-9-23-7-2-5-15(13-23)21-17(25)11-19-27;;/h1-2,4-7,10-13H,3,8-9H2,(H2-2,20,21,24,25,26,27);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013132

(CHEMBL3261986)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C18H20N6O4.2BrH/c25-17(11-19-27)21-15-5-3-9-23(13-15)7-1-2-8-24-10-4-6-16(14-24)22-18(26)12-20-28;;/h3-6,9-14H,1-2,7-8H2,(H2-2,21,22,25,26,27,28);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 5.23E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50013133

(CHEMBL3261987)Show SMILES [Br-].[Br-].O\N=C/C(=O)Nc1ccc[n+](CCCCC[n+]2cccc(NC(=O)\C=N/O)c2)c1 Show InChI InChI=1S/C19H22N6O4.2BrH/c26-18(12-20-28)22-16-6-4-10-24(14-16)8-2-1-3-9-25-11-5-7-17(15-25)23-19(27)13-21-29;;/h4-7,10-15H,1-3,8-9H2,(H2-2,22,23,26,27,28,29);2*1H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a |
Defence Research & Development Establishment (DRDE)
Curated by ChEMBL
| Assay Description
Binding affinity to sarin-inhibited hemoglobin free human erythrocyte ghost acetylcholinesterase using acetylthiocholineiodide as substrate measured ... |
Bioorg Med Chem 22: 2684-91 (2014)
Article DOI: 10.1016/j.bmc.2014.03.023
BindingDB Entry DOI: 10.7270/Q2BC413S |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024958

(2-Oxo-Propionaldehyde Oxime | Monoisonitrosoaceton...)Show InChI InChI=1S/C3H5NO2/c1-3(5)2-4-6/h2,6H,1H3/b4-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a |
Pt. Ravishankar Shukla University
Curated by ChEMBL
| Assay Description
Binding affinity to paraoxon-inhibited AChE (unknown origin) |
Bioorg Med Chem Lett 24: 4743-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.095
BindingDB Entry DOI: 10.7270/Q2RR20TJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50024959

(2,3-Butanedione Monoxime | CHEBI:4480 | CHEMBL1255...)Show InChI InChI=1S/C4H7NO2/c1-3(5-7)4(2)6/h7H,1-2H3/b5-3+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3.93E+5 | n/a | n/a | n/a | n/a | n/a |
Pt. Ravishankar Shukla University
Curated by ChEMBL
| Assay Description
Binding affinity to paraoxon-inhibited AChE (unknown origin) |
Bioorg Med Chem Lett 24: 4743-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.07.095
BindingDB Entry DOI: 10.7270/Q2RR20TJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data