

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM1250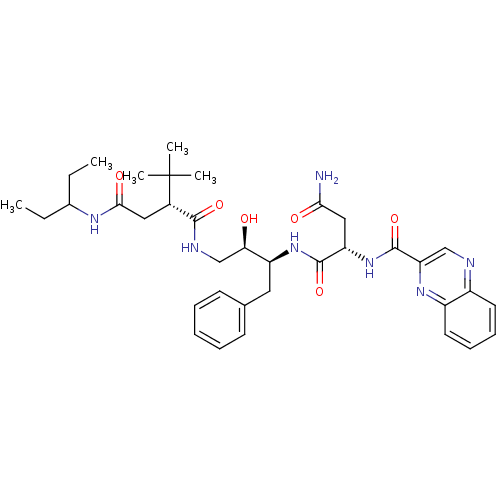 ((2S)-N-[(2S,3R)-4-[(2R)-2-tert-butyl-N-(pentan-3-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 40: 2164-76 (1997) Article DOI: 10.1021/jm9606608 BindingDB Entry DOI: 10.7270/Q2R20ZJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM1249 ((2S)-N-[(2S,3R)-4-[(2R)-2-tert-butyl-N-(pentan-3-y...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 40: 2164-76 (1997) Article DOI: 10.1021/jm9606608 BindingDB Entry DOI: 10.7270/Q2R20ZJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM729 ((2S,4R)-N-tert-butyl-1-[(2R,3S)-2-hydroxy-3-[(2S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.130 | -14.0 | 10 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 43: 1094-108 (2000) Article DOI: 10.1021/jm990336n BindingDB Entry DOI: 10.7270/Q2BZ647F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM1248 ((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S)-3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 40: 2164-76 (1997) Article DOI: 10.1021/jm9606608 BindingDB Entry DOI: 10.7270/Q2R20ZJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM1247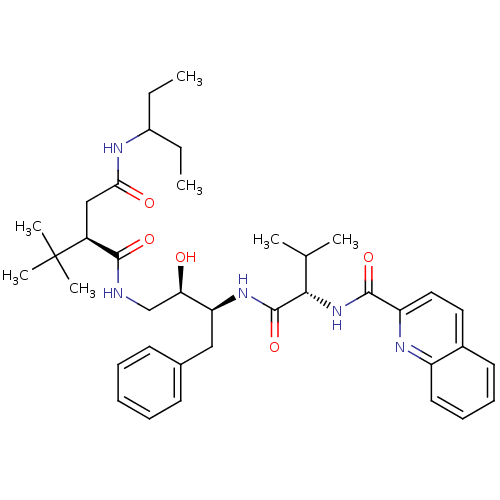 ((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S)-3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 40: 2164-76 (1997) Article DOI: 10.1021/jm9606608 BindingDB Entry DOI: 10.7270/Q2R20ZJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM1252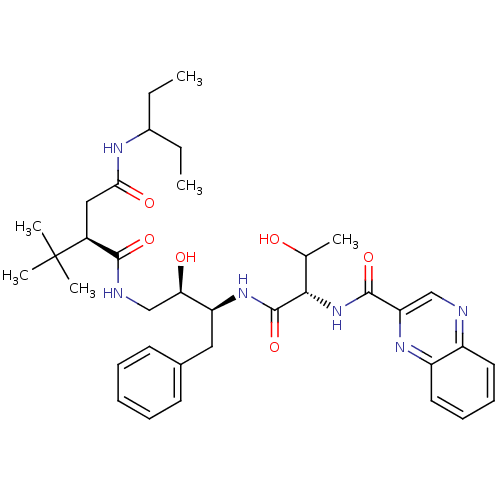 ((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S,3R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 135 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 40: 2164-76 (1997) Article DOI: 10.1021/jm9606608 BindingDB Entry DOI: 10.7270/Q2R20ZJW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [514-612] (Human immunodeficiency virus type 2) | BDBM1251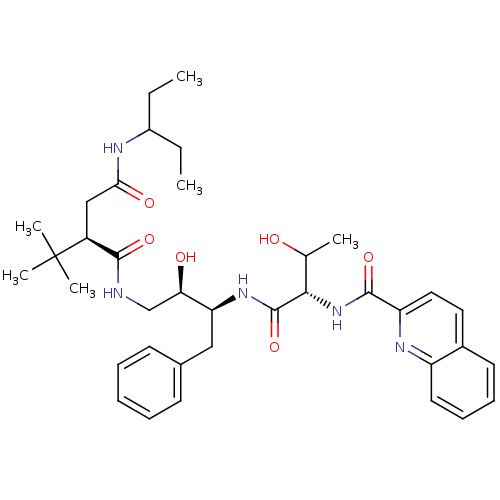 ((2R)-2-tert-butyl-N-[(2R,3S)-2-hydroxy-3-[(2S,3R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 185 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Cleavage products and substrate w... | J Med Chem 40: 2164-76 (1997) Article DOI: 10.1021/jm9606608 BindingDB Entry DOI: 10.7270/Q2R20ZJW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [613-1167,R896S,E946D,S1117V]/[618-1043,R896S,E946D] (Human immunodeficiency virus type 2) | BDBM33045 (2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
National Cancer Institute | Assay Description RNaseH assays were performed using an 18-nucleotide 3-fluorescein-labeled RNA annealed to a complementary 18-nucleotide 5-dabcyl conjugated DNA. The ... | ACS Chem Biol 3: 635-44 (2008) Article DOI: 10.1021/cb8001039 BindingDB Entry DOI: 10.7270/Q29P300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [613-1167,R896S,E946D,S1117V]/[618-1043,R896S,E946D] (Human immunodeficiency virus type 2) | BDBM33963 (vinylogous urea, NSC727448) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 20 |
National Cancer Institute | Assay Description RNaseH assays were performed using an 18-nucleotide 3-fluorescein-labeled RNA annealed to a complementary 18-nucleotide 5-dabcyl conjugated DNA. The ... | ACS Chem Biol 3: 635-44 (2008) Article DOI: 10.1021/cb8001039 BindingDB Entry DOI: 10.7270/Q29P300F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [613-1167,R896S,E946D,S1117V]/[618-1043,R896S,E946D] (Human immunodeficiency virus type 2) | BDBM2338 (Methyl 1-[(2-Amino-5-chlorophenyl)sulfonyl]-1H-pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita di Roma | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 522-30 (1996) Article DOI: 10.1021/jm950568w BindingDB Entry DOI: 10.7270/Q27W69D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [613-1167,R896S,E946D,S1117V]/[618-1043,R896S,E946D] (Human immunodeficiency virus type 2) | BDBM2339 (Pyrrolyl Aryl Sulfone (PAS) 3 | Pyrrolyl Aryl Sulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita di Roma | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 522-30 (1996) Article DOI: 10.1021/jm950568w BindingDB Entry DOI: 10.7270/Q27W69D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [613-1167,R896S,E946D,S1117V]/[618-1043,R896S,E946D] (Human immunodeficiency virus type 2) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Universita di Roma | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 39: 522-30 (1996) Article DOI: 10.1021/jm950568w BindingDB Entry DOI: 10.7270/Q27W69D4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||