

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50106497 (6-Chloro-7-(2-morpholin-4-yl-ethylamino)-quinoline...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Max-Planck-Institute of Molecular Physiology Curated by ChEMBL | Assay Description Inhibition of human Cdc25C | Bioorg Med Chem 19: 2145-55 (2011) Article DOI: 10.1016/j.bmc.2011.02.047 BindingDB Entry DOI: 10.7270/Q2BR8SHK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50504063 (CHEMBL4570006) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human CDC25C (280 to 454 residues) expressed in Escherichia coli BL21(DE3) cells using OMFP as substrate by doub... | J Med Chem 62: 7089-7110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00632 BindingDB Entry DOI: 10.7270/Q20005B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50129576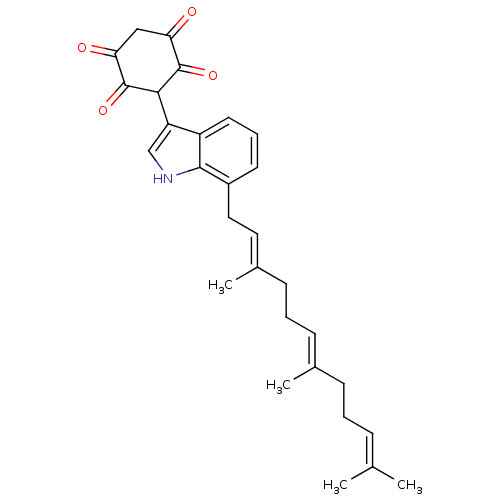 (2,5-Dihydroxy-3-[7-(3,7,11-trimethyl-dodeca-2,6,10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Inhibitory constant of compound against Cell division cycle 25 was determined | J Med Chem 46: 2580-8 (2003) Article DOI: 10.1021/jm0300835 BindingDB Entry DOI: 10.7270/Q2RJ4HVW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50504074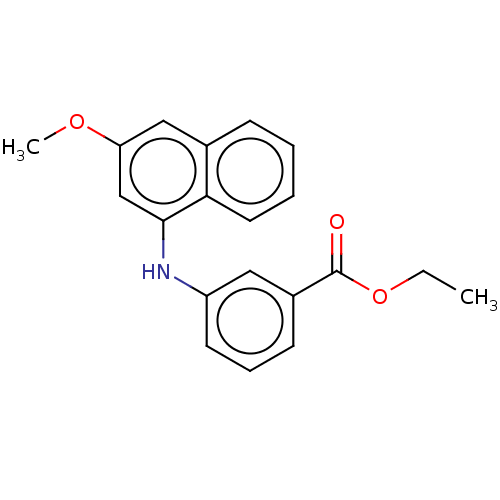 (CHEMBL4447602) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human CDC25C (280 to 454 residues) expressed in Escherichia coli BL21(DE3) cells using OMFP as substrate by doub... | J Med Chem 62: 7089-7110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00632 BindingDB Entry DOI: 10.7270/Q20005B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50131547 (2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against cell division cycle 25 degree C (Cdc25 C) was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50504077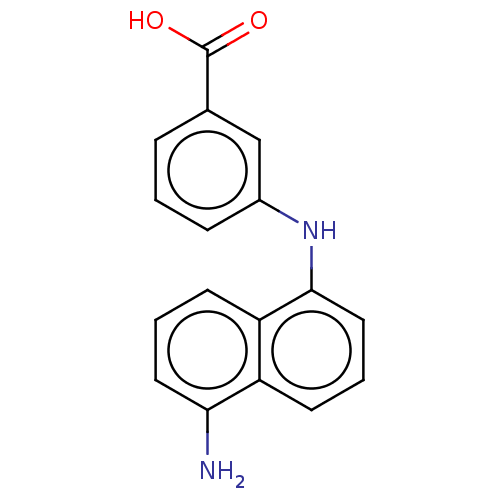 (CHEMBL4442406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Naples Federico II Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human CDC25C (280 to 454 residues) expressed in Escherichia coli BL21(DE3) cells using OMFP as substrate by doub... | J Med Chem 62: 7089-7110 (2019) Article DOI: 10.1021/acs.jmedchem.9b00632 BindingDB Entry DOI: 10.7270/Q20005B5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50132461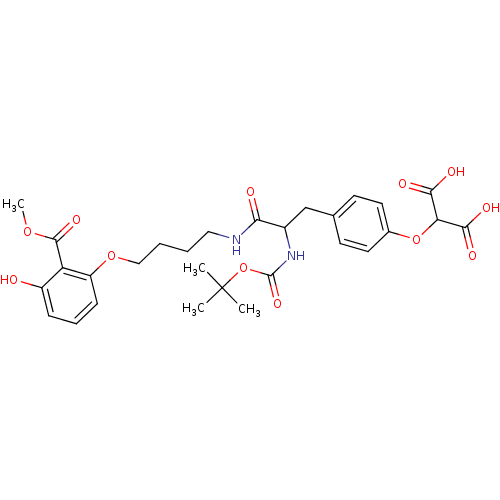 (2-(4-{2-tert-Butoxycarbonylamino-2-[4-(3-hydroxy-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory potency of the compound against Cell division cycle 25 degree C | Bioorg Med Chem Lett 13: 3129-32 (2003) BindingDB Entry DOI: 10.7270/Q24B30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50132465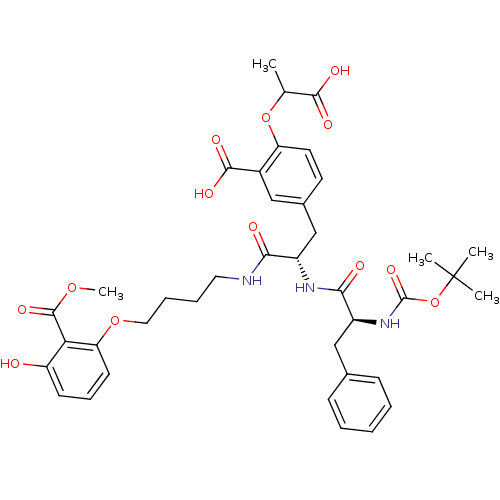 (5-{(S)-2-((S)-2-tert-Butoxycarbonylamino-3-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory potency of the compound against Cell division cycle 25 degree C | Bioorg Med Chem Lett 13: 3129-32 (2003) BindingDB Entry DOI: 10.7270/Q24B30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50132460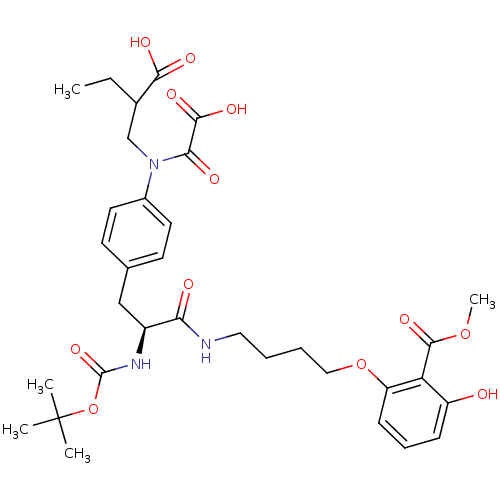 (2-[4-((S)-2-tert-Butoxycarbonylamino-3-{4-[(2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory potency of the compound against Cell division cycle 25 degree C | Bioorg Med Chem Lett 13: 3129-32 (2003) BindingDB Entry DOI: 10.7270/Q24B30QV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| M-phase inducer phosphatase 3 (Homo sapiens (Human)) | BDBM50131545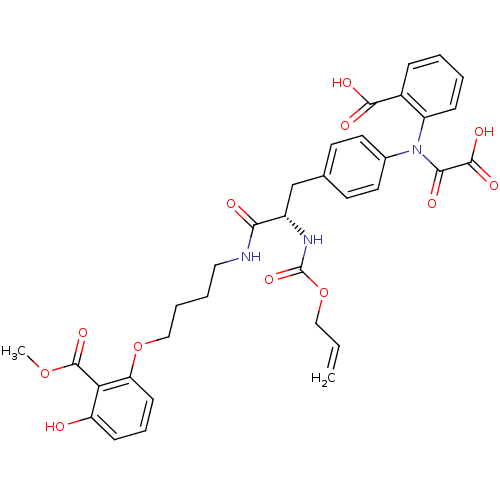 ((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibitory activity against Cell division cycle 25 degree C was determined | J Med Chem 46: 3437-40 (2003) Article DOI: 10.1021/jm034088d BindingDB Entry DOI: 10.7270/Q2WW7H1Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||