 Found 19 hits of ic50 data for polymerid = 4537
Found 19 hits of ic50 data for polymerid = 4537 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CAMK2B using KKALRRQETVDAL as substrate by [gamma-33P]-ATP assay |
Citation and Details
Article DOI: 10.1016/j.bmc.2018.02.022
BindingDB Entry DOI: 10.7270/Q2DJ5KB8 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Institute of Science and Technology (KIST)
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2b (unknown origin) |
Eur J Med Chem 141: 657-675 (2017)
Article DOI: 10.1016/j.ejmech.2017.10.003
BindingDB Entry DOI: 10.7270/Q2CV4M86 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK2B using KKALRRQETVDAL as substrate by [gamma-33P]-ATP assay |
Eur J Med Chem 161: 456-467 (2019)
Article DOI: 10.1016/j.ejmech.2018.10.052
BindingDB Entry DOI: 10.7270/Q2W380MT |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2B |
J Med Chem 52: 3191-204 (2009)
Article DOI: 10.1021/jm800861c
BindingDB Entry DOI: 10.7270/Q23J3DWT |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of CaMK2 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50359359

(CHEMBL1929238)Show SMILES CN(C)CCN1CCN(CCC1=O)C(=O)c1cc(sc1NC(=O)Nc1cccc(Cl)c1Cl)C(C)(C)C Show InChI InChI=1S/C25H33Cl2N5O3S/c1-25(2,3)19-15-16(22(36-19)29-24(35)28-18-8-6-7-17(26)21(18)27)23(34)32-10-9-20(33)31(13-14-32)12-11-30(4)5/h6-8,15H,9-14H2,1-5H3,(H2,28,29,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2beta |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50563780
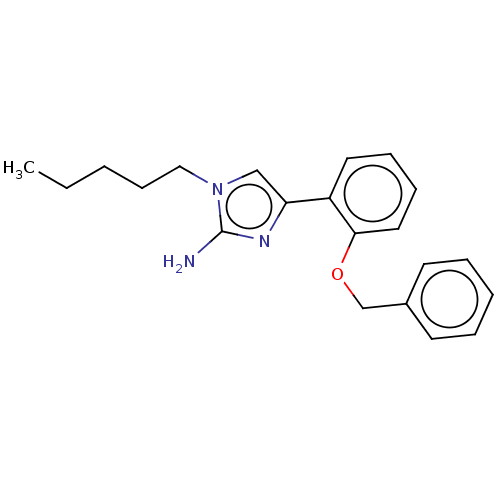
(CHEMBL4795714) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type human CAMK2beta using KKALRRQETVDAL peptide as substrate in presence of Ca2+ calmodulin and [gamma-33P]-ATP by radiometric ho... |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.6b00023
BindingDB Entry DOI: 10.7270/Q2CR5Z3F |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM7460

(2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...)Show InChI InChI=1S/C15H10O7/c16-7-4-10(19)12-11(5-7)22-15(14(21)13(12)20)6-1-2-8(17)9(18)3-6/h1-5,16-19,21H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2 using autocamtide-2 as substrate after 30 mins by PKLight assay |
Bioorg Med Chem 19: 4710-20 (2011)
Article DOI: 10.1016/j.bmc.2011.07.005
BindingDB Entry DOI: 10.7270/Q2959JM7 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50554257
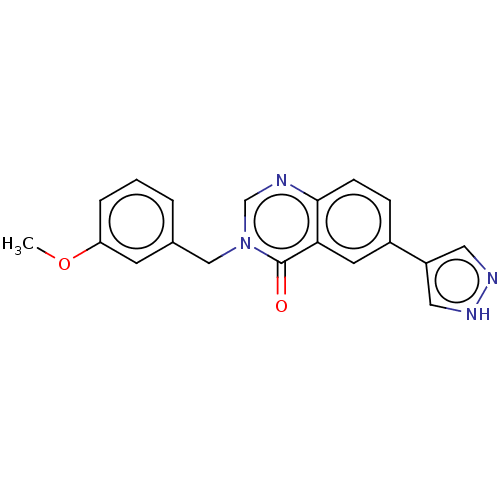
(CHEMBL4742990) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CaMK2beta |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM106870
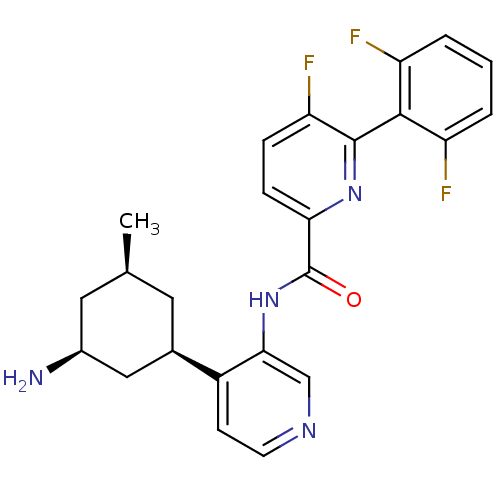
(US8592455, 70)Show SMILES C[C@@H]1C[C@H](N)C[C@@H](C1)c1ccncc1NC(=O)c1ccc(F)c(n1)-c1c(F)cccc1F |r,wU:6.8,3.3,1.0,(-.67,2.69,;-2,1.93,;-3.33,2.69,;-4.67,1.93,;-6,2.69,;-4.67,.38,;-3.33,-.38,;-2,.38,;-3.33,-1.93,;-4.67,-2.69,;-4.67,-4.23,;-3.33,-5,;-2,-4.23,;-2,-2.69,;-.67,-1.93,;.67,-2.69,;.67,-4.23,;2,-1.93,;3.33,-2.69,;4.67,-1.93,;4.67,-.38,;6,.38,;3.33,.38,;2,-.38,;3.33,1.93,;4.67,2.69,;6,1.93,;4.67,4.23,;3.33,5,;2,4.23,;2,2.69,;.67,1.93,)| Show InChI InChI=1S/C24H23F3N4O/c1-13-9-14(11-15(28)10-13)16-7-8-29-12-21(16)31-24(32)20-6-5-19(27)23(30-20)22-17(25)3-2-4-18(22)26/h2-8,12-15H,9-11,28H2,1H3,(H,31,32)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2 (unknown origin) |
J Med Chem 58: 8373-86 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01275
BindingDB Entry DOI: 10.7270/Q2H41VGN |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50499634
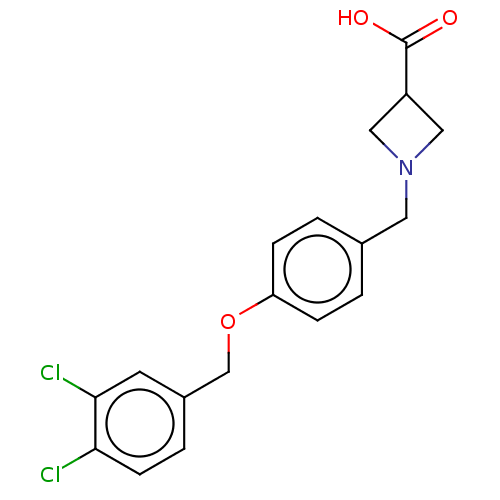
(CHEMBL3741589)Show SMILES OC(=O)C1CN(Cc2ccc(OCc3ccc(Cl)c(Cl)c3)cc2)C1 Show InChI InChI=1S/C18H17Cl2NO3/c19-16-6-3-13(7-17(16)20)11-24-15-4-1-12(2-5-15)8-21-9-14(10-21)18(22)23/h1-7,14H,8-11H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Bioresearch Center
Curated by ChEMBL
| Assay Description
Inhibition of CaMK2beta (unknown origin) |
J Med Chem 58: 9154-70 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00928
BindingDB Entry DOI: 10.7270/Q2PZ5CV4 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50135286

(CHEMBL3745885)Show SMILES Cn1c2nc(Nc3ccc4[nH]ccc4c3)ncc2cc(c1=O)S(=O)(=O)c1ccc(F)cc1F Show InChI InChI=1S/C22H15F2N5O3S/c1-29-20-13(9-19(21(29)30)33(31,32)18-5-2-14(23)10-16(18)24)11-26-22(28-20)27-15-3-4-17-12(8-15)6-7-25-17/h2-11,25H,1H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Icahn School of Medicine at Mount Sinai
Curated by ChEMBL
| Assay Description
Inhibition of human CAMK2beta using [KKALRRQETVDAL] as substrate |
Bioorg Med Chem 24: 521-44 (2016)
Article DOI: 10.1016/j.bmc.2015.11.045
BindingDB Entry DOI: 10.7270/Q24Q7WT8 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50355393

(BGJ398 | CHEMBL1834657 | US9434697, BGJ398 | US973...)Show SMILES CCN1CCN(CC1)c1ccc(Nc2cc(ncn2)N(C)C(=O)Nc2c(Cl)c(OC)cc(OC)c2Cl)cc1 Show InChI InChI=1S/C26H31Cl2N7O3/c1-5-34-10-12-35(13-11-34)18-8-6-17(7-9-18)31-21-15-22(30-16-29-21)33(2)26(36)32-25-23(27)19(37-3)14-20(38-4)24(25)28/h6-9,14-16H,5,10-13H2,1-4H3,(H,32,36)(H,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant CAMK2 |
J Med Chem 54: 7066-83 (2011)
Article DOI: 10.1021/jm2006222
BindingDB Entry DOI: 10.7270/Q22N52N1 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50519662
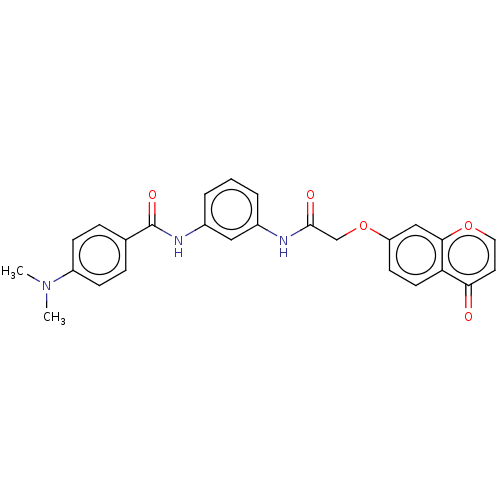
(CHEMBL4438748)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1cccc(NC(=O)COc2ccc3c(c2)occc3=O)c1 Show InChI InChI=1S/C26H23N3O5/c1-29(2)20-8-6-17(7-9-20)26(32)28-19-5-3-4-18(14-19)27-25(31)16-34-21-10-11-22-23(30)12-13-33-24(22)15-21/h3-15H,16H2,1-2H3,(H,27,31)(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human CaMK2beta (1 to 315 residues) using calmodulin a substrate incubated for 40 mins in presence of [gamma-33ATP] by radi... |
J Med Chem 62: 10691-10710 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01143
BindingDB Entry DOI: 10.7270/Q2MC93FG |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50554258
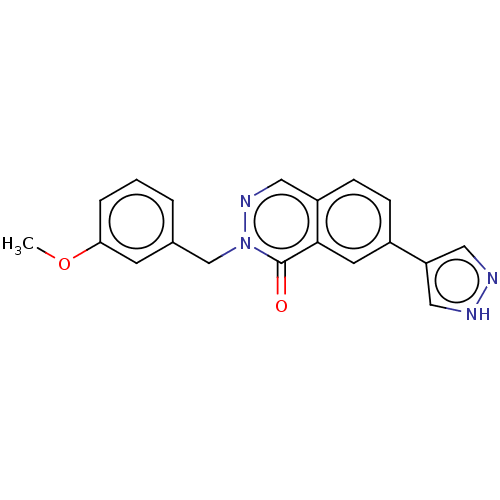
(CHEMBL4744858) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human CaMK2beta |
Citation and Details
Article DOI: 10.1016/j.bmcl.2020.127602
BindingDB Entry DOI: 10.7270/Q2H135PS |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50558812
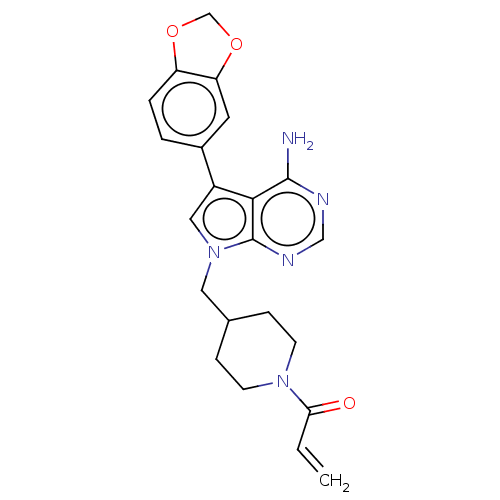
(CHEMBL4755698)Show SMILES Nc1ncnc2n(CC3CCN(CC3)C(=O)C=C)cc(-c3ccc4OCOc4c3)c12 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CAMK2 (unknown origin) |
Citation and Details
Article DOI: 10.1016/j.ejmech.2017.12.079
BindingDB Entry DOI: 10.7270/Q2P272TR |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50388936
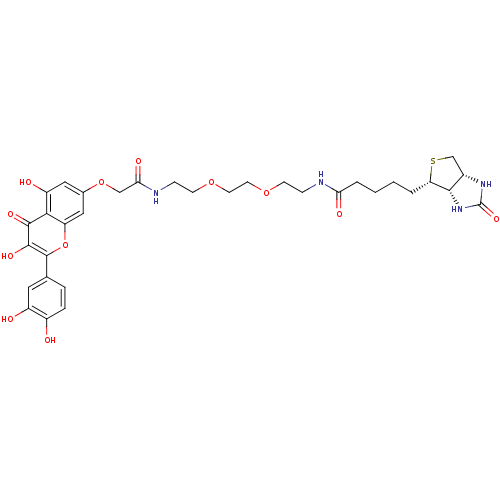
(CHEMBL2063416)Show SMILES Oc1ccc(cc1O)-c1oc2cc(OCC(=O)NCCOCCOCCNC(=O)CCCC[C@@H]3SC[C@@H]4NC(=O)N[C@H]34)cc(O)c2c(=O)c1O |r| Show InChI InChI=1S/C33H40N4O12S/c38-21-6-5-18(13-22(21)39)32-31(44)30(43)28-23(40)14-19(15-24(28)49-32)48-16-27(42)35-8-10-47-12-11-46-9-7-34-26(41)4-2-1-3-25-29-20(17-50-25)36-33(45)37-29/h5-6,13-15,20,25,29,38-40,44H,1-4,7-12,16-17H2,(H,34,41)(H,35,42)(H2,36,37,45)/t20-,25-,29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2 using autocamtide-2 as substrate after 30 mins by PKLight assay |
Bioorg Med Chem 19: 4710-20 (2011)
Article DOI: 10.1016/j.bmc.2011.07.005
BindingDB Entry DOI: 10.7270/Q2959JM7 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ansaris
Curated by ChEMBL
| Assay Description
Inhibition of CAMK2beta |
Bioorg Med Chem Lett 21: 7155-65 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.078
BindingDB Entry DOI: 10.7270/Q2NC61NH |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50537742
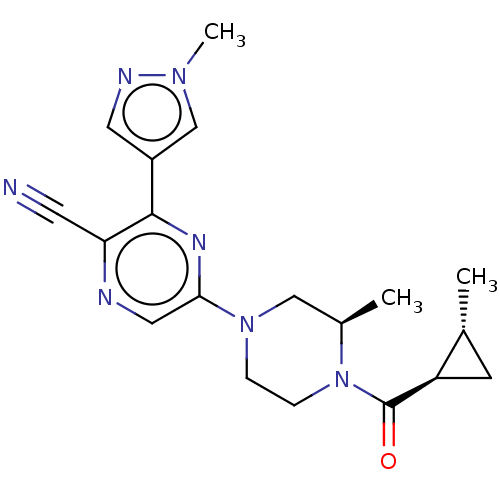
(CHEMBL4634634 | US11179389, Compound 1-14)Show SMILES C[C@@H]1C[C@H]1C(=O)N1CCN(C[C@H]1C)c1cnc(C#N)c(n1)-c1cnn(C)c1 |r| Show InChI InChI=1S/C19H23N7O/c1-12-6-15(12)19(27)26-5-4-25(10-13(26)2)17-9-21-16(7-20)18(23-17)14-8-22-24(3)11-14/h8-9,11-13,15H,4-6,10H2,1-3H3/t12-,13-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]8-OH-DPAT binding to 5-hydroxytryptamine 1A receptor in rat cerebral cortex membranes |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126715
BindingDB Entry DOI: 10.7270/Q2HM5CZG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data