

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50108052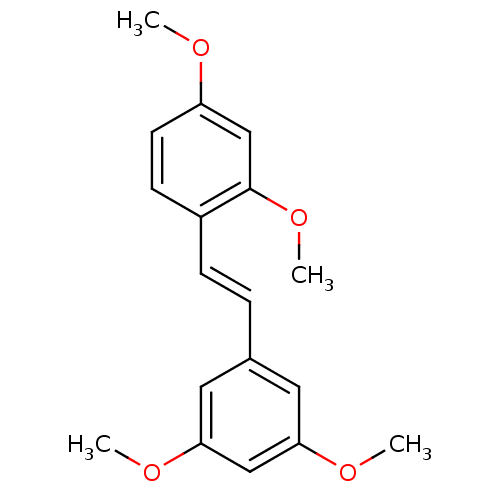 (1-[(E)-2-(3,5-dimethoxyphenyl)vinyl]-2,4-dimethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 using ethoxyresorufin as substrate preincubated for 3 mins followed by NADPH addition measured after 10 mins by EROD assay | Eur J Med Chem 163: 28-36 (2019) Article DOI: 10.1016/j.ejmech.2018.11.039 BindingDB Entry DOI: 10.7270/Q2930XFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23409 (3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of human liver microsomes CYP1B1 expressed in baculovirus infected insect cells coexpressing human NADPH-cytochrome P450 reductase using 7... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50252405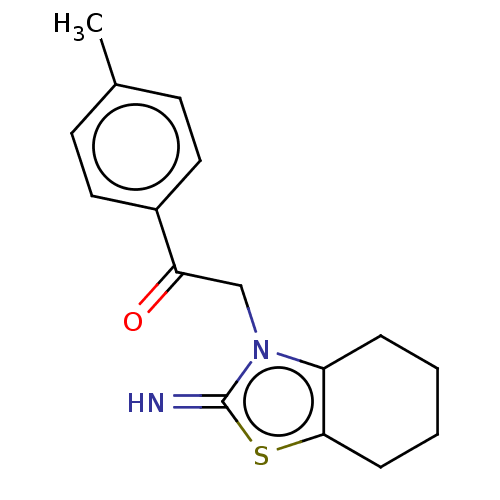 (CHEMBL556353 | Pifithrin-Alpha) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 in TCDD-exposed human MCF-7 cells using ethoxyresorufin as substrate after 20 mins in presence of NADPH by Lineweaver-Burk linea... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50432672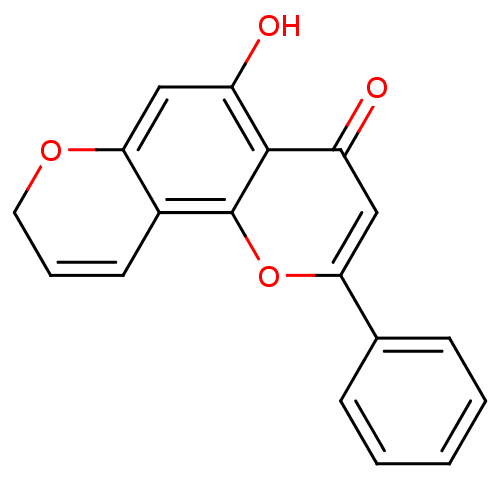 (CHEMBL2347912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23415 (5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23415 (5,7-dihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23414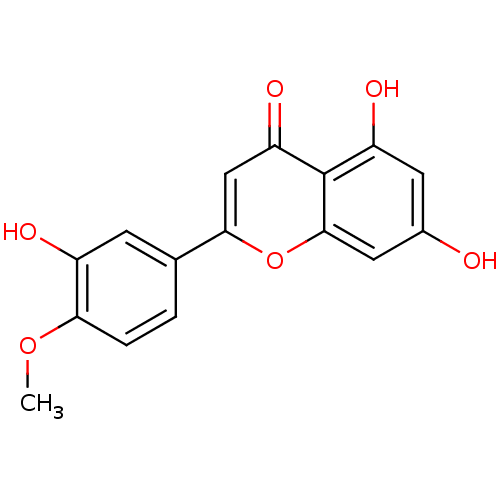 (5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23414 (5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7461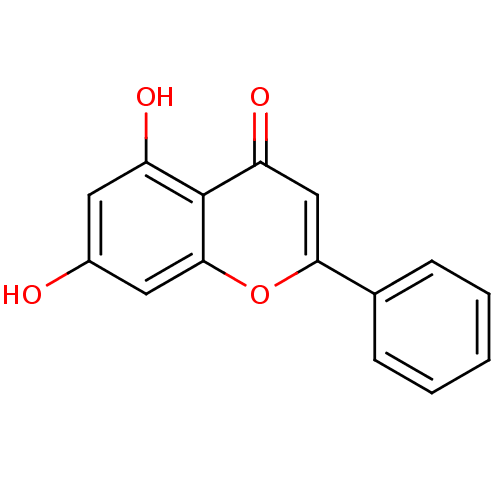 (5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7461 (5,7-dihydroxy-2-phenyl-4H-chromen-4-one | 5,7-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM15236 (3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50390428 (CHEMBL2071344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant CYP1B1 (unknown origin) expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50344054 (3',5-dihydroxy-4',6,7-trimethoxy flavone | 6-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 (unknown origin) | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50344054 (3',5-dihydroxy-4',6,7-trimethoxy flavone | 6-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50390427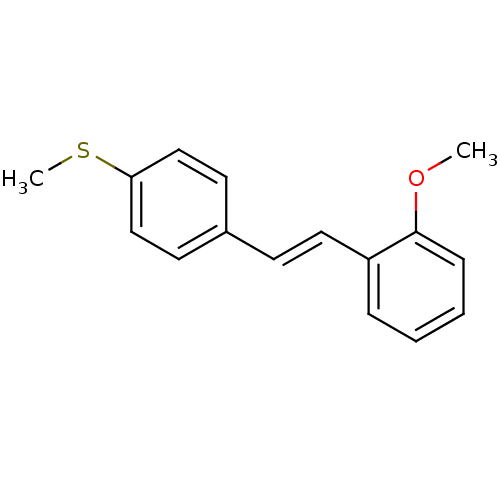 (CHEMBL2071343) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant CYP1B1 (unknown origin) expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7462 (3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50203126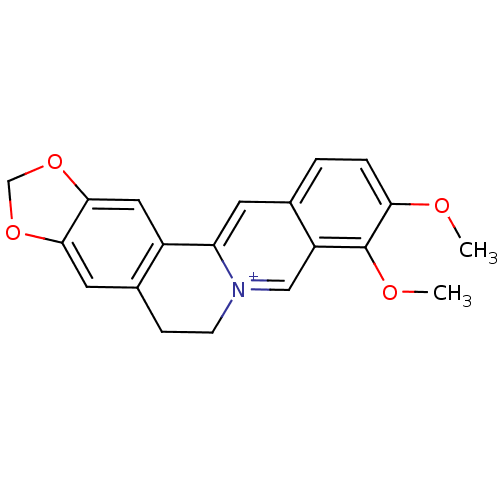 (3,4-dimethoxy-6,7-dihydro-[1,3]dioxolo[4,5-g]pyrid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in Escherichia coli DH5alpha coexpressing human NADPH-P450 reductase using 4-estradiol as substrate in presence ... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50432677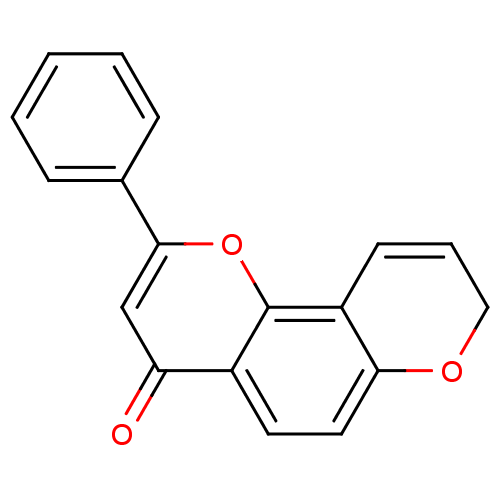 (CHEMBL2347756) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50045924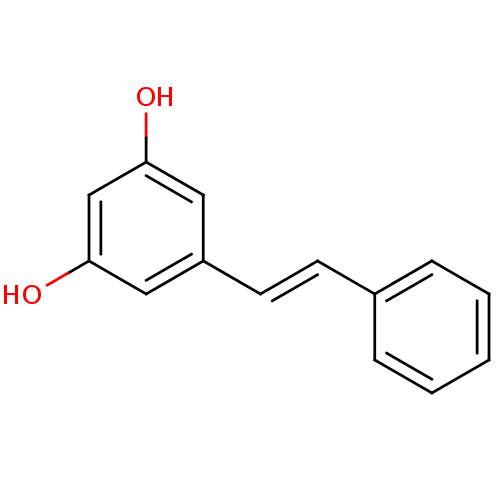 ((E)-3,5-stilbenediol | (E)-5-(2-phenylethenyl)-1,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 74.3 | -10.1 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Tokyo | Assay Description The ethoxyresorufin-O-deethylase (EROD) assay is used to test the activity of CYP1B1. | Chem Biol 14: 613-21 (2007) Article DOI: 10.1016/j.chembiol.2007.05.004 BindingDB Entry DOI: 10.7270/Q2S46QDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50113260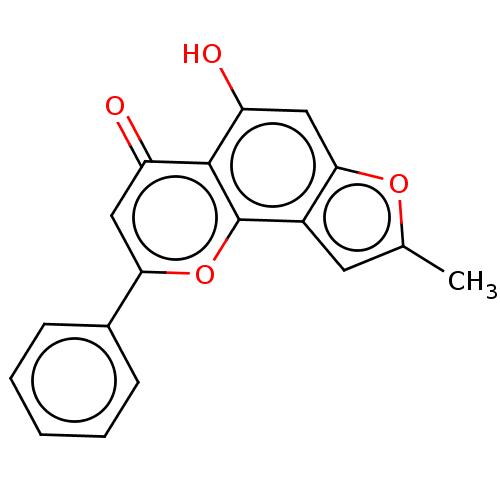 (CHEMBL3601434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50113261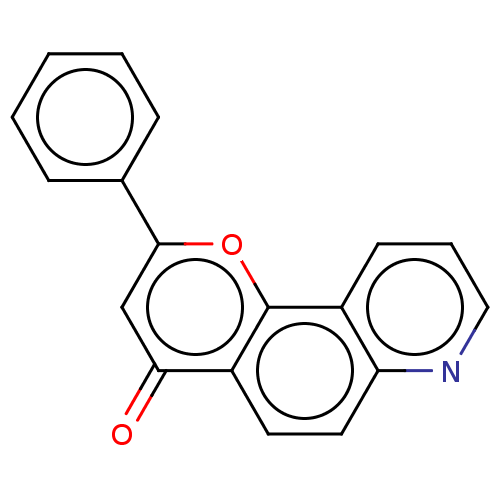 (CHEMBL3601435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50061117 (6,6,9-Trimethyl-3-pentyl-6H-benzo[c]chromen-1-ol |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Competitive inhibition of human liver microsomes CYP1B1 expressed in supersomes coexpressing NADPH-CYP reductase using 7-Ethoxyresorufin as substrate... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 177 | -9.58 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Tokyo | Assay Description The ethoxyresorufin-O-deethylase (EROD) assay is used to test the activity of CYP1B1. | Chem Biol 14: 613-21 (2007) Article DOI: 10.1016/j.chembiol.2007.05.004 BindingDB Entry DOI: 10.7270/Q2S46QDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50113259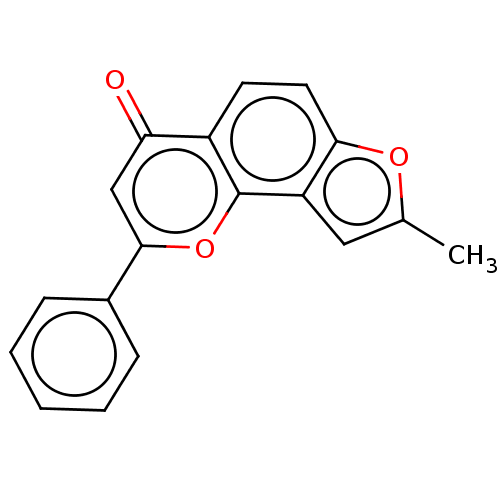 (CHEMBL3601433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23411 (5,6,7-trihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50009001 (5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50390427 (CHEMBL2071343) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant CYP1B1 (unknown origin) expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50247272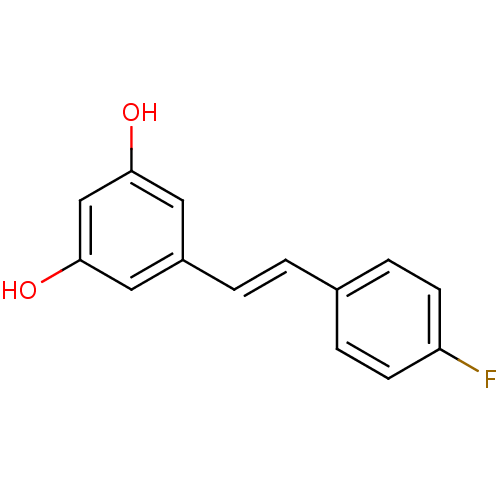 (5-(4-fluorostyryl)benzene-1,3-diol | CHEMBL451311 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 323 | -9.20 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Tokyo | Assay Description The ethoxyresorufin-O-deethylase (EROD) assay is used to test the activity of CYP1B1. | Chem Biol 14: 613-21 (2007) Article DOI: 10.1016/j.chembiol.2007.05.004 BindingDB Entry DOI: 10.7270/Q2S46QDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50113262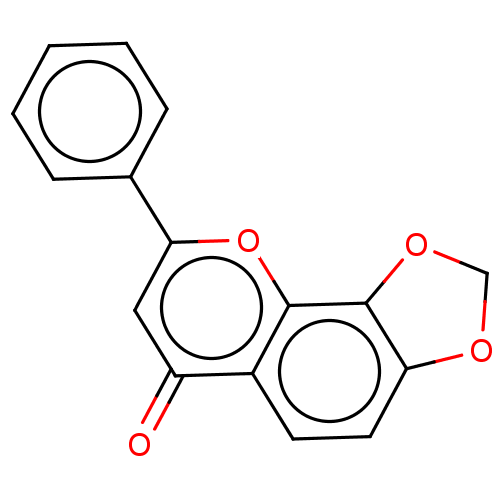 (CHEMBL3601436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50206434 (1,2-Anthraquinonediol | 1,2-dihydroxy-9,10-anthraq...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli coexpressing human NADPH-cytochrome P450 reductase using 7-ethoxyresorufin as su... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50390428 (CHEMBL2071344) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant CYP1B1 (unknown origin) expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50134461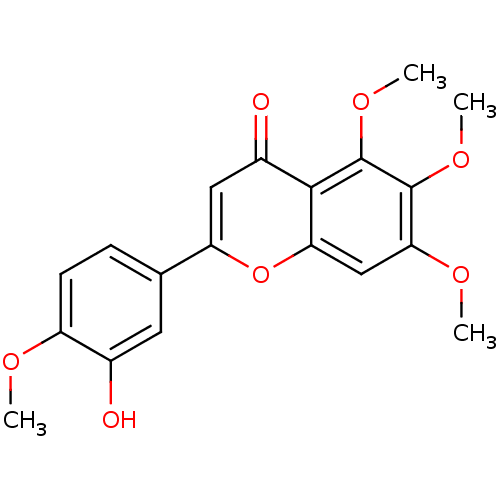 (2-(3-Hydroxy-4-methoxy-phenyl)-5,6,7-trimethoxy-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM67454 (1,2,4-trihydroxy-9,10-anthraquinone | 1,2,4-trihyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in Escherichia coli coexpressing human NADPH-cytochrome P450 reductase using 7-ethoxyresorufin as su... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 750 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Birla Institute of Technology Curated by ChEMBL | Assay Description Mixed type inhibition of human CYP1B1 by EROD assay | Eur J Med Chem 163: 28-36 (2019) Article DOI: 10.1016/j.ejmech.2018.11.039 BindingDB Entry DOI: 10.7270/Q2930XFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM23926 ((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after 15 mins in... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50025321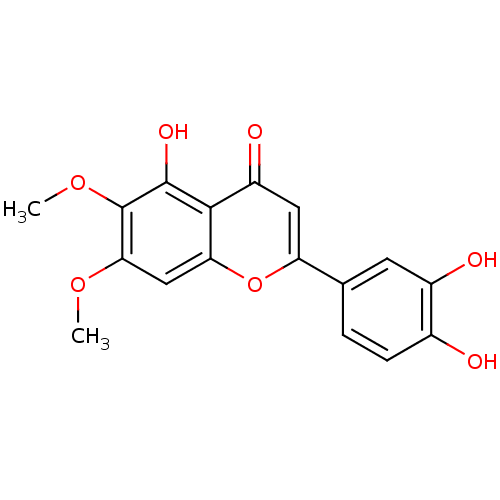 (2-(3,4-Dihydroxy-phenyl)-5-hydroxy-6,7-dimethoxy-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | >800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete Curated by ChEMBL | Assay Description Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin | Bioorg Med Chem 19: 2842-9 (2011) Article DOI: 10.1016/j.bmc.2011.03.042 BindingDB Entry DOI: 10.7270/Q2V69JXK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50252422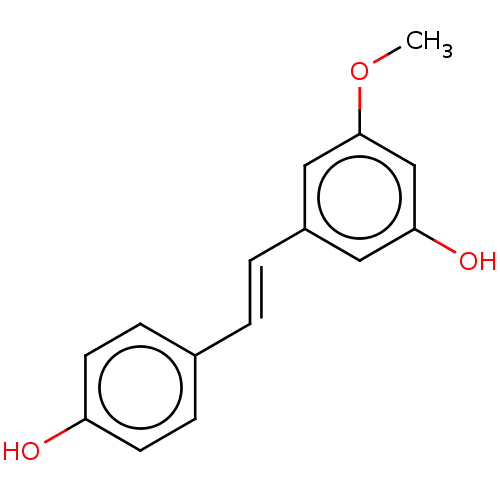 (CHEBI:63672 | CHEMBL498917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after 15 mins in... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50131688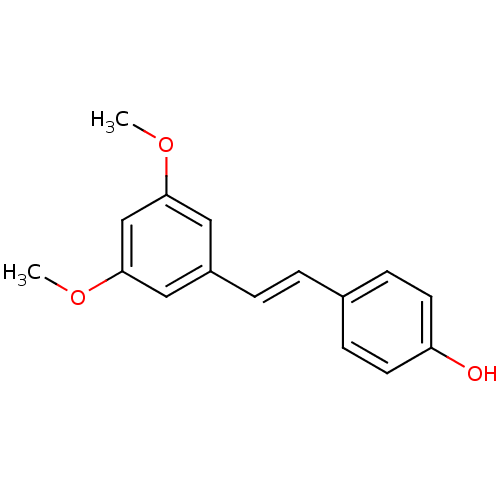 ((E)-4-(3,5-dimethoxystyryl)phenol | 3,5-Dimethoxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in supersomes coexpressing NADPH-CYP reductase using 7-ethoxyresorufin as substrate after 15 mins in... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50308719 ((9-[(3-methyl-2-buten-1-yl)oxy]-7H-furo[3,2-g][1]b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Competitive inhibition of CYP1B1 (unknown origin) expressed in yeast microsomes using (-)benzo[a]pyrene-7R-trans-7,8-dihyrodiol (B[a]P-7,8-diol) as s... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM68279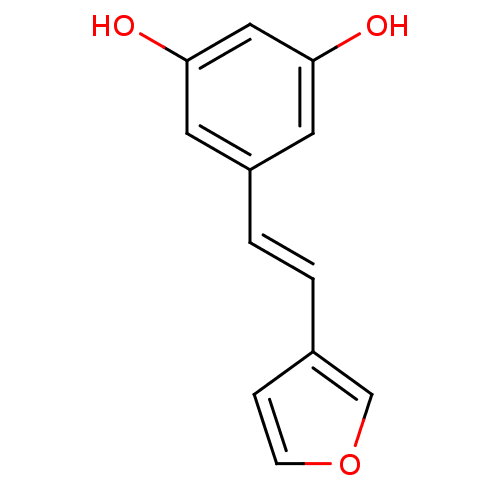 (Stilbene, 15f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | -8.44 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Tokyo | Assay Description The ethoxyresorufin-O-deethylase (EROD) assay is used to test the activity of CYP1B1. | Chem Biol 14: 613-21 (2007) Article DOI: 10.1016/j.chembiol.2007.05.004 BindingDB Entry DOI: 10.7270/Q2S46QDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM68280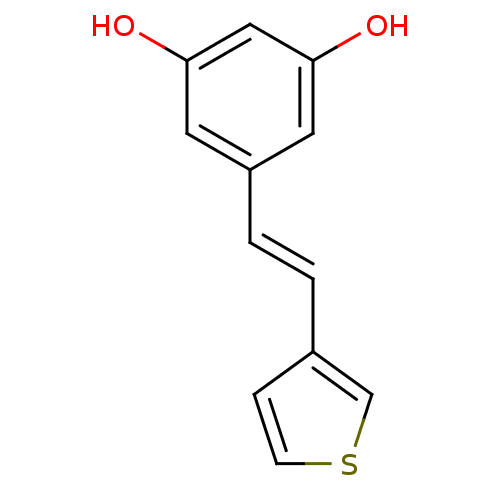 (Stilbene, 16f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.18E+3 | -8.41 | n/a | n/a | n/a | n/a | n/a | 8.0 | 37 |
University of Tokyo | Assay Description The ethoxyresorufin-O-deethylase (EROD) assay is used to test the activity of CYP1B1. | Chem Biol 14: 613-21 (2007) Article DOI: 10.1016/j.chembiol.2007.05.004 BindingDB Entry DOI: 10.7270/Q2S46QDG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM17292 ((1S,10R,11S,14S,15S)-15-methyltetracyclo[8.7.0.0^{...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of human CYP1B1 expressed in supersomes using ethoxyresorufin as substrate after 20 mins in presence of NADP by Dixon plot analysis | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM9461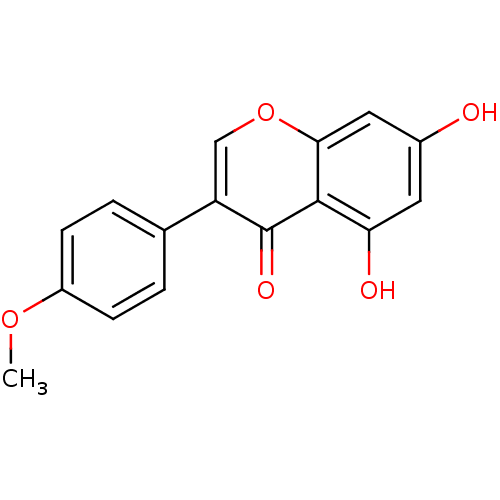 (5,7-dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of CYP1B1 in human liver microsomes coexpressing recombinant human cytochrome P450 oxidoreductase using 7-ethoxyresorufin as substrate aft... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50252404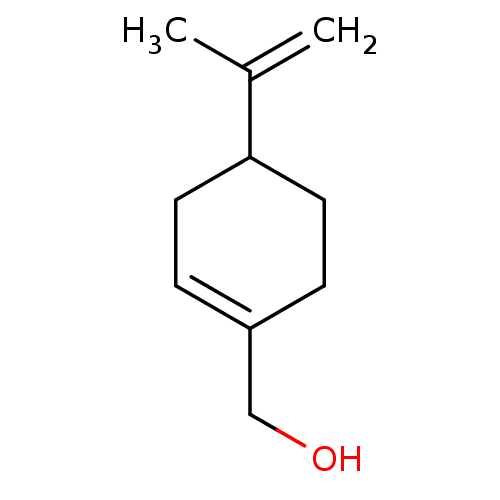 (CHEBI:15420 | CHEMBL444711) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratory of Medicinal Chemistry, Endocrinology and Nephrology Unit, CHU de Québec - Research Center, Québec, Québec, Canada; Department of Molecular Medicine, Faculty of Medicine, Université Laval, Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1B1 expressed in supersomes using ethoxyresorufin as substrate after 20 mins in presence of NADPH by fluorescence ... | Eur J Med Chem 135: 296-306 (2017) Article DOI: 10.1016/j.ejmech.2017.04.042 BindingDB Entry DOI: 10.7270/Q26Q20QW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 76 total ) | Next | Last >> |