

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039564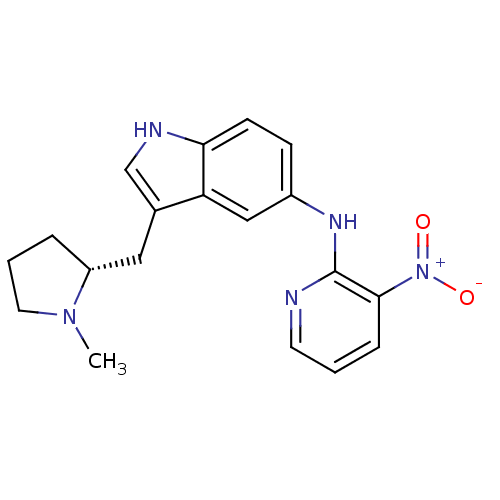 (CHEMBL83597 | [3-((R)-1-Methyl-pyrrolidin-2-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of forskolin stimulated adenylate cyclase at 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039566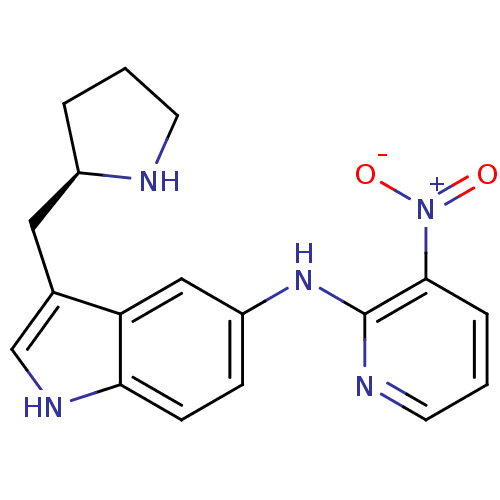 ((3-Nitro-pyridin-2-yl)-(3-(R)-1-pyrrolidin-2-ylmet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of forskolin stimulated adenylate cyclase at 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039567 (CHEMBL84942 | [3-(1-Methyl-pyrrolidin-3-yl)-1H-ind...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of forskolin stimulated adenylate cyclase at 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50039565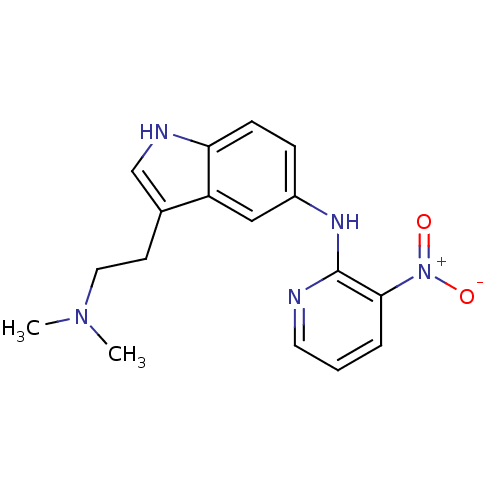 (CHEMBL314213 | [3-(2-Dimethylamino-ethyl)-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of forskolin stimulated adenylate cyclase at 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM10755 (14C-5-hydroxy tryptamine creatinine disulfate | 2-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of forskolin stimulated adenylate cyclase at 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | 97 | n/a | n/a | n/a | n/a |
Pfizer Inc Curated by ChEMBL | Assay Description Inhibition of forskolin stimulated adenylate cyclase at 5-hydroxytryptamine 1D receptor | J Med Chem 37: 2509-12 (1994) BindingDB Entry DOI: 10.7270/Q2S181JQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (RAT) | BDBM84737 (3-(1,2,3,6-Tetrahydro-pyridin-4-yl)-1,4-dihydro-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | PubMed | n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of Forskolin-stimulated adenylate cyclase activity against 5-hydroxytryptamine 1D receptor of rat substantia nigra | J Med Chem 33: 2087-93 (1990) BindingDB Entry DOI: 10.7270/Q2GQ6WR3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||