 Found 68 hits of ic50 for UniProtKB: P35355
Found 68 hits of ic50 for UniProtKB: P35355 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(RAT) | BDBM50289087
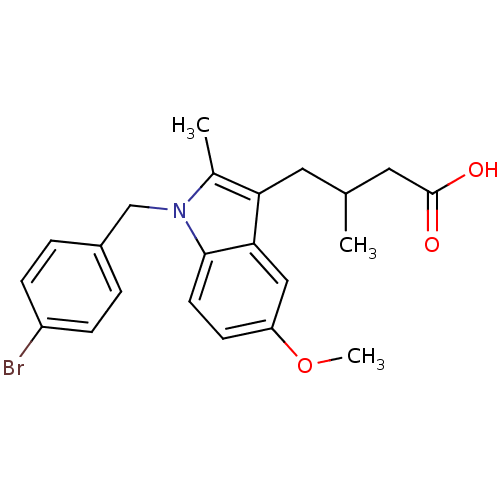
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289082
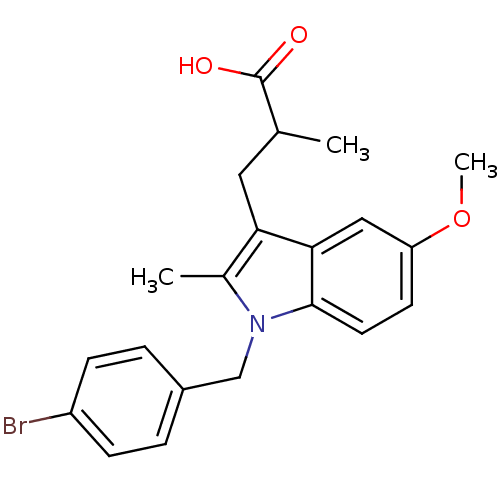
(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-13(21(24)25)10-18-14(2)23(12-15-4-6-16(22)7-5-15)20-9-8-17(26-3)11-19(18)20/h4-9,11,13H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289084
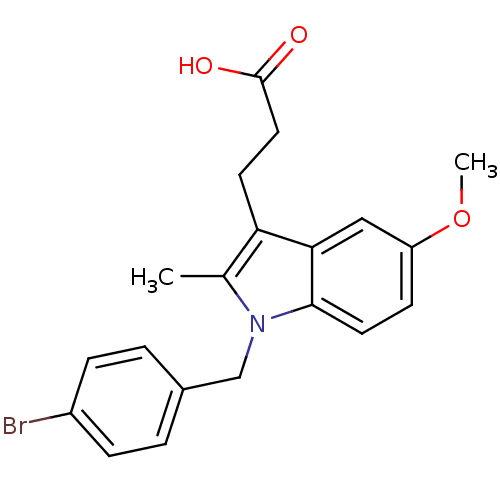
(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCC(O)=O)c2c1 Show InChI InChI=1S/C20H20BrNO3/c1-13-17(8-10-20(23)24)18-11-16(25-2)7-9-19(18)22(13)12-14-3-5-15(21)6-4-14/h3-7,9,11H,8,10,12H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289083

(3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(C(C)CC(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-13(10-20(24)25)21-14(2)23(12-15-4-6-16(22)7-5-15)19-9-8-17(26-3)11-18(19)21/h4-9,11,13H,10,12H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289088
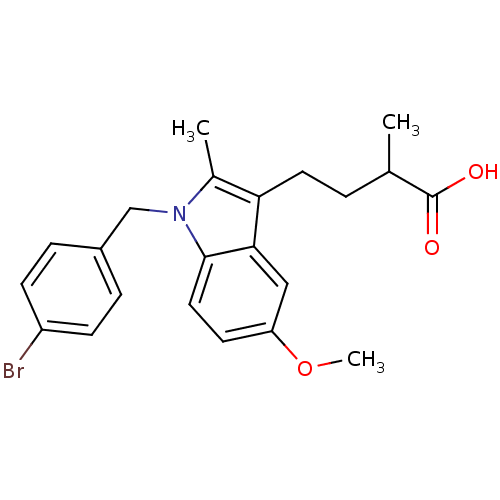
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCC(C)C(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(22(25)26)4-10-19-15(2)24(13-16-5-7-17(23)8-6-16)21-11-9-18(27-3)12-20(19)21/h5-9,11-12,14H,4,10,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289081
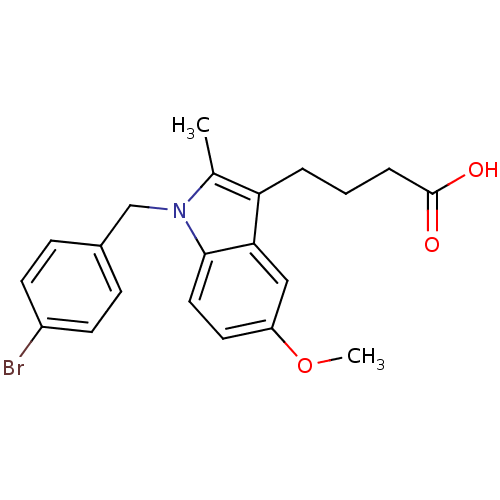
(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CCCC(O)=O)c2c1 Show InChI InChI=1S/C21H22BrNO3/c1-14-18(4-3-5-21(24)25)19-12-17(26-2)10-11-20(19)23(14)13-15-6-8-16(22)9-7-15/h6-12H,3-5,13H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM22970
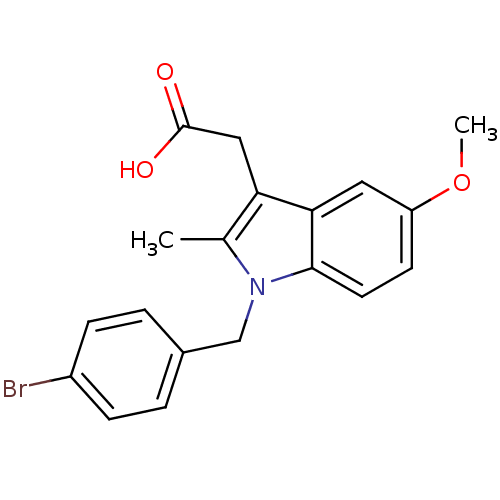
(2-{1-[(4-bromophenyl)methyl]-5-methoxy-2-methyl-1H...)Show InChI InChI=1S/C19H18BrNO3/c1-12-16(10-19(22)23)17-9-15(24-2)7-8-18(17)21(12)11-13-3-5-14(20)6-4-13/h3-9H,10-11H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289090
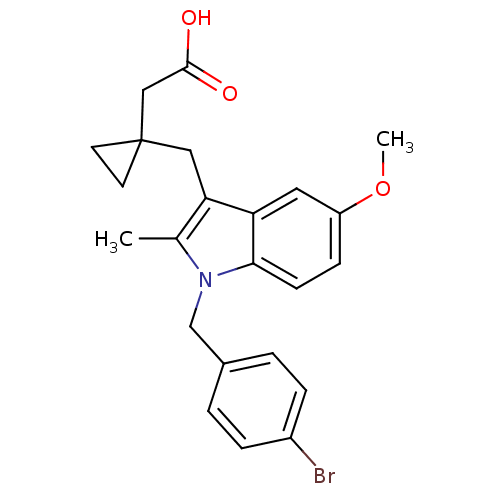
(CHEMBL162776 | {1-[1-(4-Bromo-benzyl)-5-methoxy-2-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC3(CC(O)=O)CC3)c2c1 Show InChI InChI=1S/C23H24BrNO3/c1-15-20(12-23(9-10-23)13-22(26)27)19-11-18(28-2)7-8-21(19)25(15)14-16-3-5-17(24)6-4-16/h3-8,11H,9-10,12-14H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289089
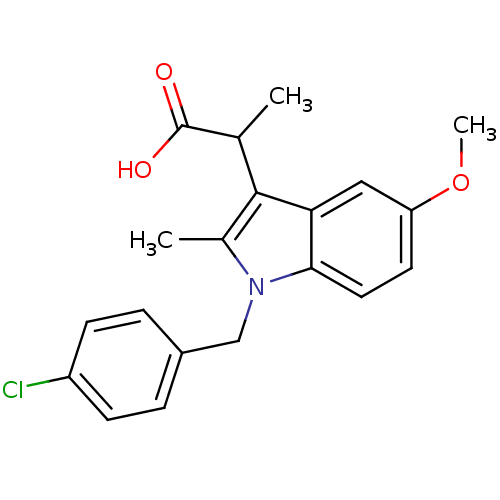
(2-[1-(4-Chloro-benzyl)-5-methoxy-2-methyl-1H-indol...)Show SMILES COc1ccc2n(Cc3ccc(Cl)cc3)c(C)c(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C20H20ClNO3/c1-12(20(23)24)19-13(2)22(11-14-4-6-15(21)7-5-14)18-9-8-16(25-3)10-17(18)19/h4-10,12H,11H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289091

((S)-4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-in...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(C[C@H](C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260464

(CHEMBL4071383)Show SMILES CN1C=CN(C=C1)C1NC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C15H16F3N5O2S/c1-21-6-8-22(9-7-21)14-20-13(15(16,17)18)10-23(14)11-2-4-12(5-3-11)26(19,24)25/h2-10,14,20H,1H3,(H2,19,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289087

(4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-indol-...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(CC(C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260465

(CHEMBL4101003)Show SMILES CN1C=CC(C=C1)C1OC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C16H16F3N3O3S/c1-21-8-6-11(7-9-21)15-22(10-14(25-15)16(17,18)19)12-2-4-13(5-3-12)26(20,23)24/h2-11,15H,1H3,(H2,20,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289092
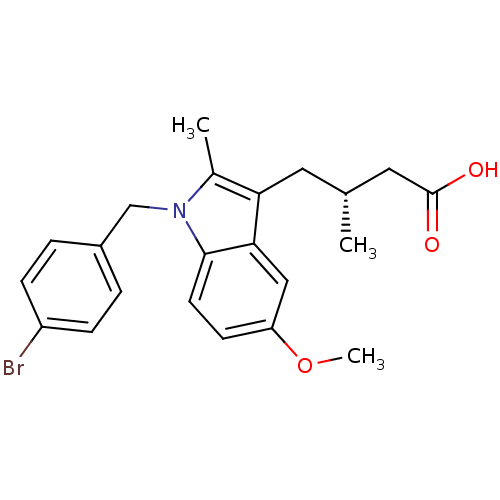
((R)-4-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1H-in...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c(C[C@@H](C)CC(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-14(11-22(25)26)10-19-15(2)24(13-16-4-6-17(23)7-5-16)21-9-8-18(27-3)12-20(19)21/h4-9,12,14H,10-11,13H2,1-3H3,(H,25,26)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated in vitro for inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 731-736 (1996)
Article DOI: 10.1016/0960-894X(96)00101-1
BindingDB Entry DOI: 10.7270/Q21J99RF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289086
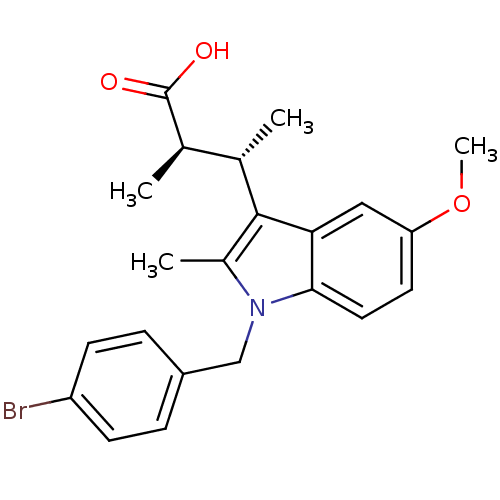
((2R,3S)-3-[1-(4-Bromo-benzyl)-5-methoxy-2-methyl-1...)Show SMILES COc1ccc2n(Cc3ccc(Br)cc3)c(C)c([C@@H](C)[C@@H](C)C(O)=O)c2c1 Show InChI InChI=1S/C22H24BrNO3/c1-13(14(2)22(25)26)21-15(3)24(12-16-5-7-17(23)8-6-16)20-10-9-18(27-4)11-19(20)21/h5-11,13-14H,12H2,1-4H3,(H,25,26)/t13-,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260467
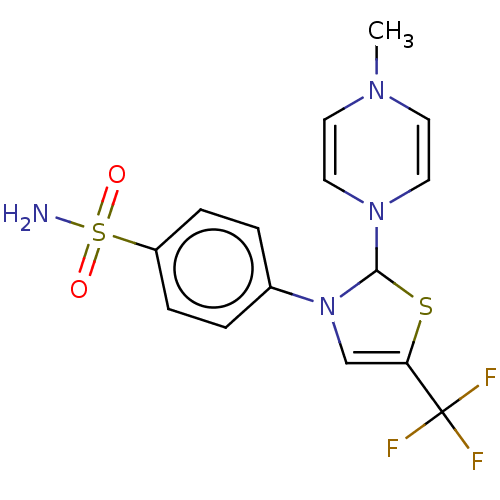
(CHEMBL4101946)Show SMILES CN1C=CN(C=C1)C1SC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C15H15F3N4O2S2/c1-20-6-8-21(9-7-20)14-22(10-13(25-14)15(16,17)18)11-2-4-12(5-3-11)26(19,23)24/h2-10,14H,1H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260470
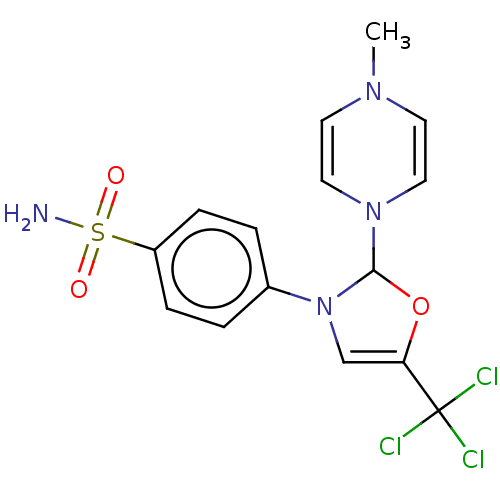
(CHEMBL4063353)Show SMILES CN1C=CN(C=C1)C1OC(=CN1c1ccc(cc1)S(N)(=O)=O)C(Cl)(Cl)Cl |c:2,5,10| Show InChI InChI=1S/C15H15Cl3N4O3S/c1-20-6-8-21(9-7-20)14-22(10-13(25-14)15(16,17)18)11-2-4-12(5-3-11)26(19,23)24/h2-10,14H,1H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260472
(CHEMBL4061498)Show SMILES CN1C=CC(C=C1)C1NC(C)=CN1c1ccc(cc1)S(N)(=O)=O |c:2,5,11| Show InChI InChI=1S/C16H20N4O2S/c1-12-11-20(14-3-5-15(6-4-14)23(17,21)22)16(18-12)13-7-9-19(2)10-8-13/h3-11,13,16,18H,1-2H3,(H2,17,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50369676
(CHEMBL4161423)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(SCCN(O)C(N)=O)nn1-c1ccc(Br)cc1 Show InChI InChI=1S/C18H18BrN5O4S2/c1-30(27,28)15-8-2-12(3-9-15)16-21-18(29-11-10-23(26)17(20)25)22-24(16)14-6-4-13(19)5-7-14/h2-9,26H,10-11H2,1H3,(H2,20,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105290

(2-[7-Methoxy-4-(4-methyl-benzyl)-naphthalen-1-yl]-...)Show InChI InChI=1S/C22H22O3/c1-14-4-6-16(7-5-14)12-17-8-10-19(15(2)22(23)24)21-13-18(25-3)9-11-20(17)21/h4-11,13,15H,12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105302
(2-[7-Methoxy-4-(4-methoxy-benzyl)-naphthalen-1-yl]...)Show SMILES COc1ccc(Cc2ccc(C(C)C(O)=O)c3cc(OC)ccc23)cc1 Show InChI InChI=1S/C22H22O4/c1-14(22(23)24)19-10-6-16(12-15-4-7-17(25-2)8-5-15)20-11-9-18(26-3)13-21(19)20/h4-11,13-14H,12H2,1-3H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105293
(2-(4-Benzyl-7-methoxy-naphthalen-1-yl)-propionic a...)Show InChI InChI=1S/C21H20O3/c1-14(21(22)23)18-10-8-16(12-15-6-4-3-5-7-15)19-11-9-17(24-2)13-20(18)19/h3-11,13-14H,12H2,1-2H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50537507
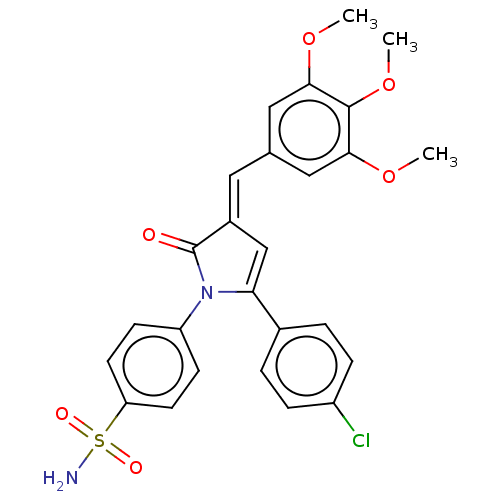
(CHEMBL4638485)Show SMILES COc1cc(\C=C2/C=C(N(C2=O)c2ccc(cc2)S(N)(=O)=O)c2ccc(Cl)cc2)cc(OC)c1OC |c:7| Show InChI InChI=1S/C26H23ClN2O6S/c1-33-23-13-16(14-24(34-2)25(23)35-3)12-18-15-22(17-4-6-19(27)7-5-17)29(26(18)30)20-8-10-21(11-9-20)36(28,31)32/h4-15H,1-3H3,(H2,28,31,32)/b18-12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C eta expressed in Sf-9 cells |
Eur J Med Chem 162: 679-734 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.017
BindingDB Entry DOI: 10.7270/Q21839DX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50289085

(CHEMBL159315 | L-748780 | [5-Methoxy-2-methyl-1-(2...)Show SMILES COc1ccc2n(C(=O)c3c(Cl)cc(Cl)cc3Cl)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H14Cl3NO4/c1-9-12(8-17(24)25)13-7-11(27-2)3-4-16(13)23(9)19(26)18-14(21)5-10(20)6-15(18)22/h3-7H,8H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 6: 725-730 (1996)
Article DOI: 10.1016/0960-894X(96)00100-X
BindingDB Entry DOI: 10.7270/Q25B02GQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105303
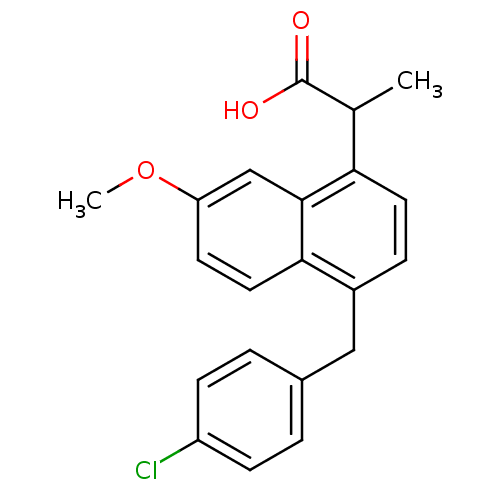
(2-[4-(4-Chloro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES COc1ccc2c(Cc3ccc(Cl)cc3)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H19ClO3/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 520 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50487330

(CHEMBL4638767)Show SMILES NS(=O)(=O)c1ccc(cc1)N1C(=O)\C(=C\c2ccc(cc2)C(F)(F)F)C=C1c1ccc(Cl)cc1 |c:27| Show InChI InChI=1S/C27H31NO2S2/c29-19-7-14-26-25(17-15-23-11-5-2-6-12-23)28-27(32-26)18-16-24(30)21-31-20-8-13-22-9-3-1-4-10-22/h1-6,9-12,16,18,29H,7-8,13-15,17,19-21H2/b18-16+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology
Curated by ChEMBL
| Assay Description
Tested for inhibition of cGMP-dependent protein kinase from bovine lung |
Eur J Med Chem 162: 679-734 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.017
BindingDB Entry DOI: 10.7270/Q21839DX |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105289
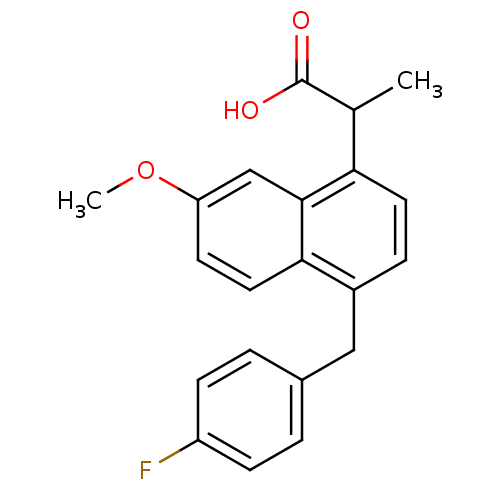
(2-[4-(4-Fluoro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show InChI InChI=1S/C21H19FO3/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM22369

(4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...)Show SMILES CS(=O)(=O)c1ccc(cc1)C1=C(C(=O)OC1)c1ccccc1 |t:11| Show InChI InChI=1S/C17H14O4S/c1-22(19,20)14-9-7-12(8-10-14)15-11-21-17(18)16(15)13-5-3-2-4-6-13/h2-10H,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105288
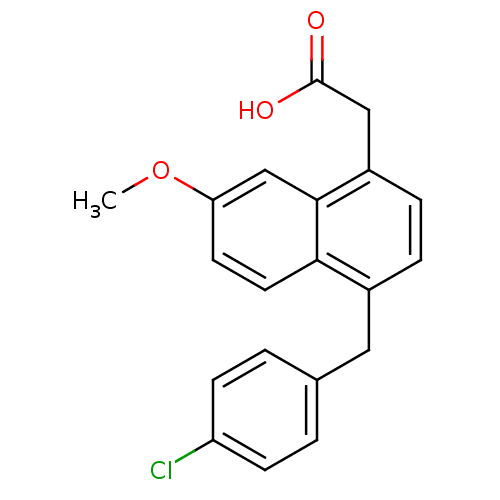
(CHEMBL91139 | [4-(4-Chloro-benzyl)-7-methoxy-napht...)Show InChI InChI=1S/C20H17ClO3/c1-24-17-8-9-18-14(10-13-2-6-16(21)7-3-13)4-5-15(11-20(22)23)19(18)12-17/h2-9,12H,10-11H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105282
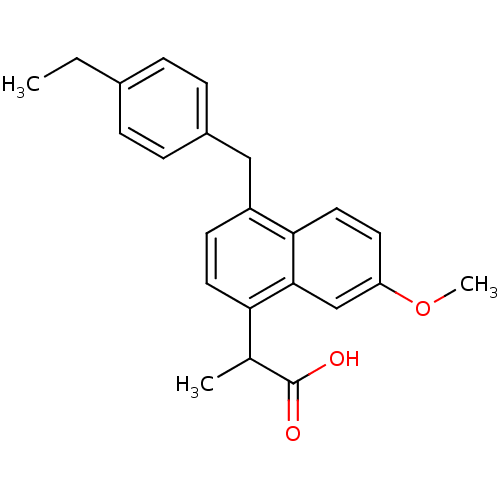
(2-[4-(4-Ethyl-benzyl)-7-methoxy-naphthalen-1-yl]-p...)Show SMILES CCc1ccc(Cc2ccc(C(C)C(O)=O)c3cc(OC)ccc23)cc1 Show InChI InChI=1S/C23H24O3/c1-4-16-5-7-17(8-6-16)13-18-9-11-20(15(2)23(24)25)22-14-19(26-3)10-12-21(18)22/h5-12,14-15H,4,13H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260477

(CHEMBL4083020)Show SMILES CN1C=CN(C=C1)C1OC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C15H15F3N4O3S/c1-20-6-8-21(9-7-20)14-22(10-13(25-14)15(16,17)18)11-2-4-12(5-3-11)26(19,23)24/h2-10,14H,1H3,(H2,19,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260462
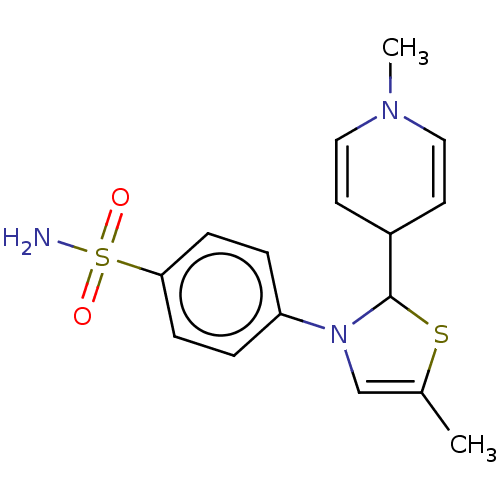
(CHEMBL4071496)Show SMILES CN1C=CC(C=C1)C1SC(C)=CN1c1ccc(cc1)S(N)(=O)=O |c:2,5,11| Show InChI InChI=1S/C16H19N3O2S2/c1-12-11-19(14-3-5-15(6-4-14)23(17,20)21)16(22-12)13-7-9-18(2)10-8-13/h3-11,13,16H,1-2H3,(H2,17,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105298
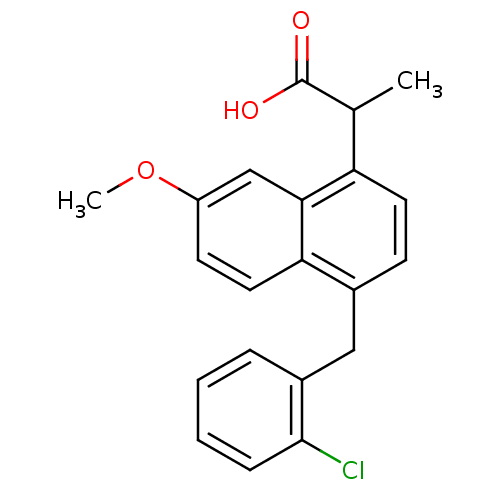
(2-[4-(2-Chloro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show InChI InChI=1S/C21H19ClO3/c1-13(21(23)24)17-9-7-14(11-15-5-3-4-6-20(15)22)18-10-8-16(25-2)12-19(17)18/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105300
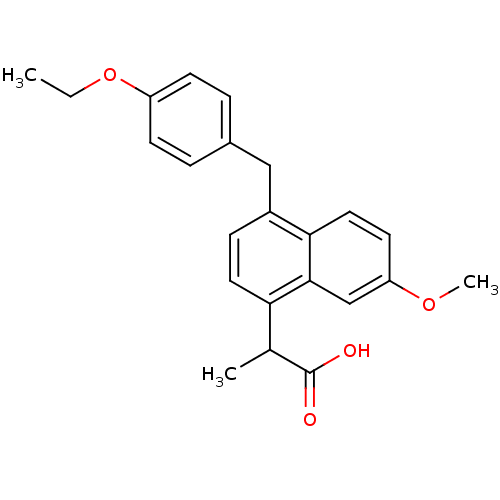
(2-[4-(4-Ethoxy-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES CCOc1ccc(Cc2ccc(C(C)C(O)=O)c3cc(OC)ccc23)cc1 Show InChI InChI=1S/C23H24O4/c1-4-27-18-8-5-16(6-9-18)13-17-7-11-20(15(2)23(24)25)22-14-19(26-3)10-12-21(17)22/h5-12,14-15H,4,13H2,1-3H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260476

(CHEMBL4093268)Show SMILES CN1C=CC(C=C1)C1NC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C16H17F3N4O2S/c1-22-8-6-11(7-9-22)15-21-14(16(17,18)19)10-23(15)12-2-4-13(5-3-12)26(20,24)25/h2-11,15,21H,1H3,(H2,20,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105286

(2-[4-(4-Fluoro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES CCC(C(O)=O)c1ccc(Cc2ccc(F)cc2)c2ccc(OC)cc12 Show InChI InChI=1S/C22H21FO3/c1-3-18(22(24)25)20-10-6-15(12-14-4-7-16(23)8-5-14)19-11-9-17(26-2)13-21(19)20/h4-11,13,18H,3,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105284
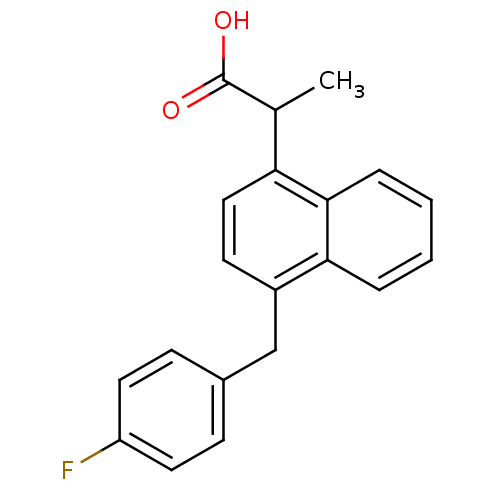
(2-[4-(4-Fluoro-benzyl)-naphthalen-1-yl]-propionic ...)Show InChI InChI=1S/C20H17FO2/c1-13(20(22)23)17-11-8-15(18-4-2-3-5-19(17)18)12-14-6-9-16(21)10-7-14/h2-11,13H,12H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105285

(2-[4-(4-Chloro-benzyl)-7-methylsulfanyl-naphthalen...)Show SMILES CSc1ccc2c(Cc3ccc(Cl)cc3)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C21H19ClO2S/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(25-2)12-20(18)19/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105287
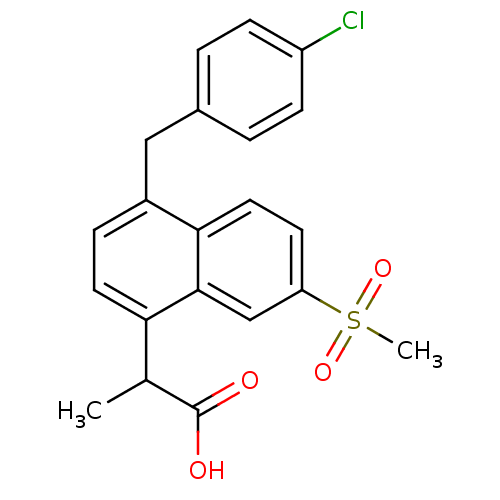
(2-[4-(4-Chloro-benzyl)-7-methanesulfonyl-naphthale...)Show SMILES CC(C(O)=O)c1ccc(Cc2ccc(Cl)cc2)c2ccc(cc12)S(C)(=O)=O Show InChI InChI=1S/C21H19ClO4S/c1-13(21(23)24)18-9-5-15(11-14-3-6-16(22)7-4-14)19-10-8-17(12-20(18)19)27(2,25)26/h3-10,12-13H,11H2,1-2H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105294
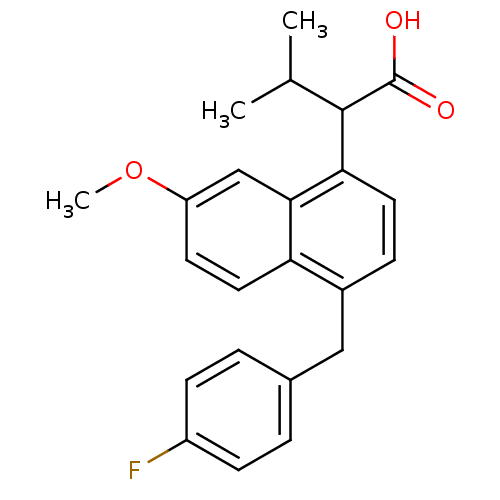
(2-[4-(4-Fluoro-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES COc1ccc2c(Cc3ccc(F)cc3)ccc(C(C(C)C)C(O)=O)c2c1 Show InChI InChI=1S/C23H23FO3/c1-14(2)22(23(25)26)20-10-6-16(12-15-4-7-17(24)8-5-15)19-11-9-18(27-3)13-21(19)20/h4-11,13-14,22H,12H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105297
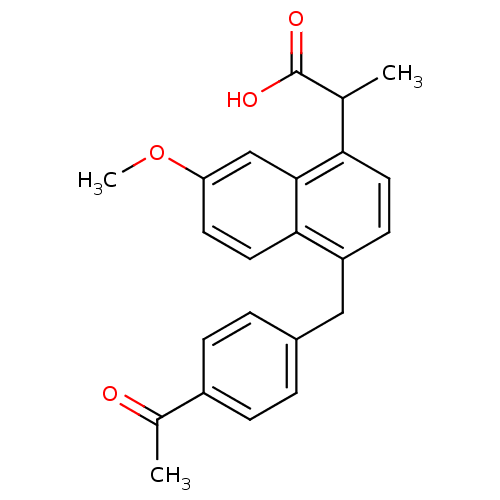
(2-[4-(4-Acetyl-benzyl)-7-methoxy-naphthalen-1-yl]-...)Show SMILES COc1ccc2c(Cc3ccc(cc3)C(C)=O)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C23H22O4/c1-14(23(25)26)20-10-8-18(21-11-9-19(27-3)13-22(20)21)12-16-4-6-17(7-5-16)15(2)24/h4-11,13-14H,12H2,1-3H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105301
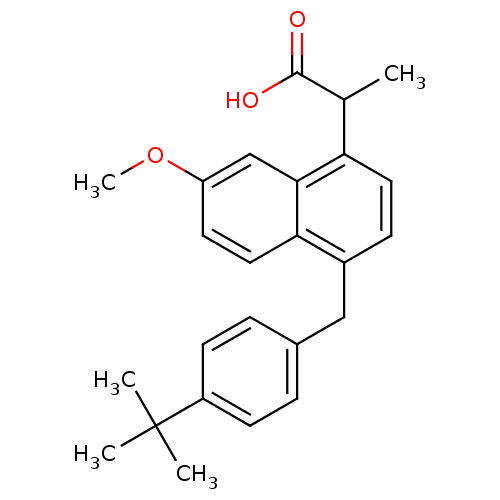
(2-[4-(4-tert-Butyl-benzyl)-7-methoxy-naphthalen-1-...)Show SMILES COc1ccc2c(Cc3ccc(cc3)C(C)(C)C)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C25H28O3/c1-16(24(26)27)21-12-8-18(22-13-11-20(28-5)15-23(21)22)14-17-6-9-19(10-7-17)25(2,3)4/h6-13,15-16H,14H2,1-5H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50260466
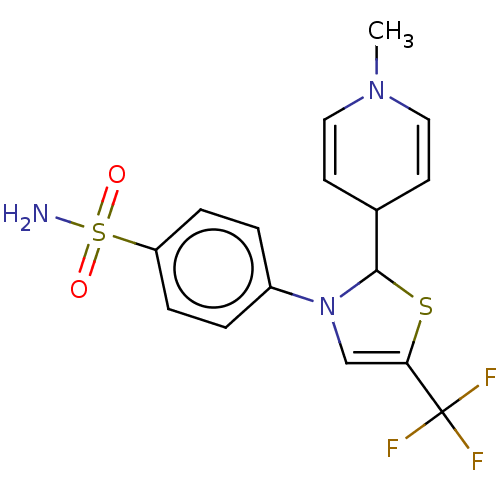
(CHEMBL4077676)Show SMILES CN1C=CC(C=C1)C1SC(=CN1c1ccc(cc1)S(N)(=O)=O)C(F)(F)F |c:2,5,10| Show InChI InChI=1S/C16H16F3N3O2S2/c1-21-8-6-11(7-9-21)15-22(10-14(25-15)16(17,18)19)12-2-4-13(5-3-12)26(20,23)24/h2-11,15H,1H3,(H2,20,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chongqing Normal University
Curated by ChEMBL
| Assay Description
Inhibition of COX-2 in rat peritoneal macrophages assessed as reduction in PGE2 production using radiolabelled-arachidonic acid as substrate pretreat... |
Bioorg Med Chem 25: 4887-4893 (2017)
Article DOI: 10.1016/j.bmc.2017.07.038
BindingDB Entry DOI: 10.7270/Q2KW5JGN |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50105281
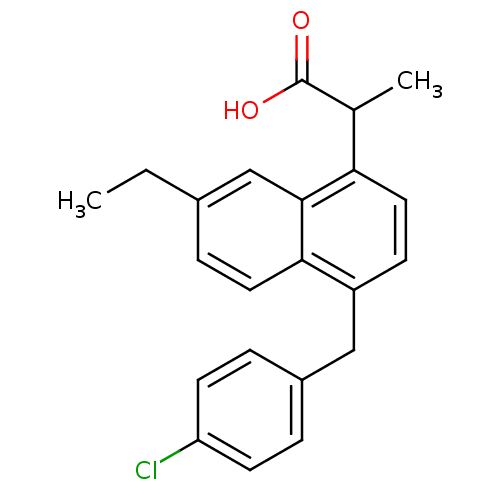
(2-[4-(4-Chloro-benzyl)-7-ethyl-naphthalen-1-yl]-pr...)Show SMILES CCc1ccc2c(Cc3ccc(Cl)cc3)ccc(C(C)C(O)=O)c2c1 Show InChI InChI=1S/C22H21ClO2/c1-3-15-6-10-20-17(12-16-4-8-18(23)9-5-16)7-11-19(21(20)13-15)14(2)22(24)25/h4-11,13-14H,3,12H2,1-2H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against rat Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 2687-90 (2001)
BindingDB Entry DOI: 10.7270/Q2BC3XV1 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(RAT) | BDBM50537508

(CHEMBL4638260)Show SMILES C\C(=N/NC(=O)CNC(=O)c1cccc(c1)S(=O)(=O)Nc1ccccc1Cl)c1ccc(F)cc1 Show InChI InChI=1S/C23H20ClFN4O4S/c1-15(16-9-11-18(25)12-10-16)27-28-22(30)14-26-23(31)17-5-4-6-19(13-17)34(32,33)29-21-8-3-2-7-20(21)24/h2-13,29H,14H2,1H3,(H,26,31)(H,28,30)/b27-15+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha expressed in Sf-9 cells |
Eur J Med Chem 162: 679-734 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.017
BindingDB Entry DOI: 10.7270/Q21839DX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data