

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50399430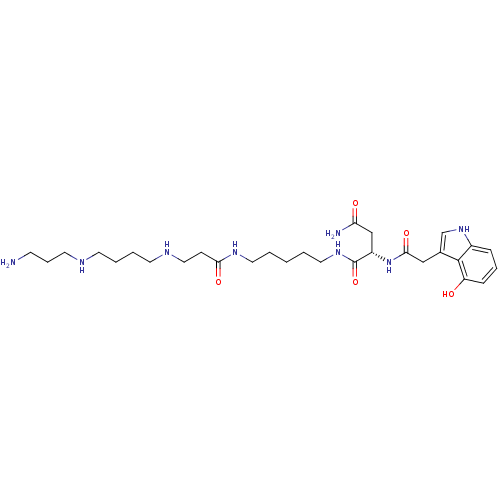 (CHEMBL2178920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes by two-electrode voltage-clamp at membrane potential -60 to -80 mV electrophys... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50399431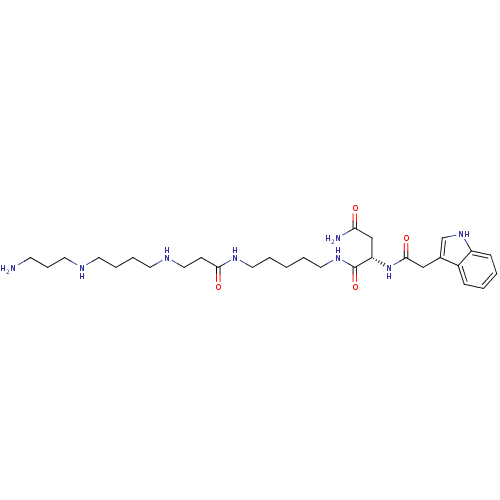 (CHEMBL2178919) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes by two-electrode voltage-clamp at membrane potential -60 to -80 mV electrophys... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50399430 (CHEMBL2178920) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrophysiology ... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50399431 (CHEMBL2178919) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrophysiology ... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50252920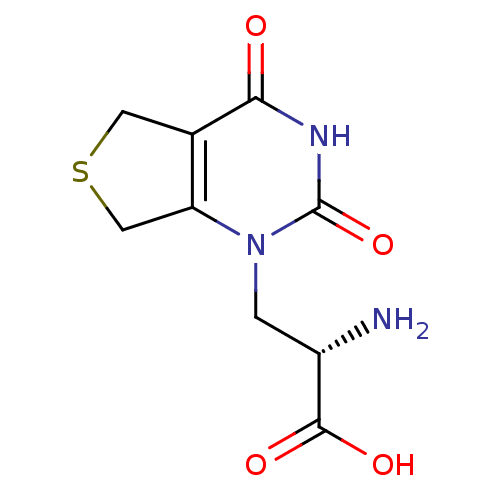 ((S)-1-[2'-Amino-2'-carboxyethyl]-5,7-dihydrothieno...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat recombinant iGluR5(Q)1b expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50252922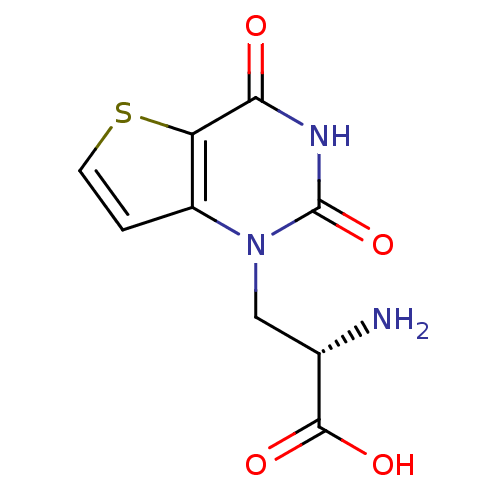 ((S)-1-(2'-Amino-2'-carboxyethyl)thieno[3,2-d]pyrim...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
European Research Centre for Drug Discovery and Development (NatSynDrugs) Curated by ChEMBL | Assay Description Displacement of [3H]SYM2081 from rat recombinant iGluR5(Q)1b expressed in Sf9 cells | J Med Chem 51: 6614-8 (2008) Article DOI: 10.1021/jm800865a BindingDB Entry DOI: 10.7270/Q261117R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50105843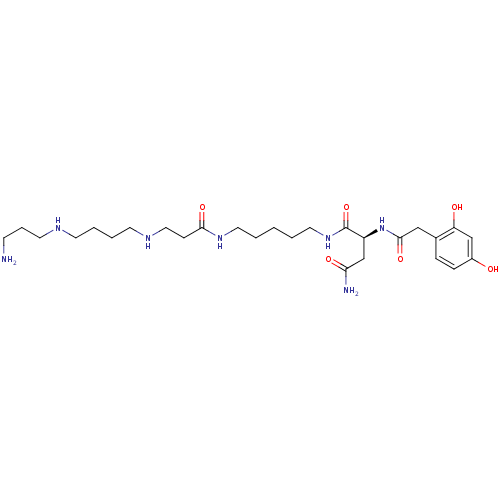 (2-Amino-N-{3-[4-(3-amino-propylamino)-butylamino]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrophysiology ... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50002369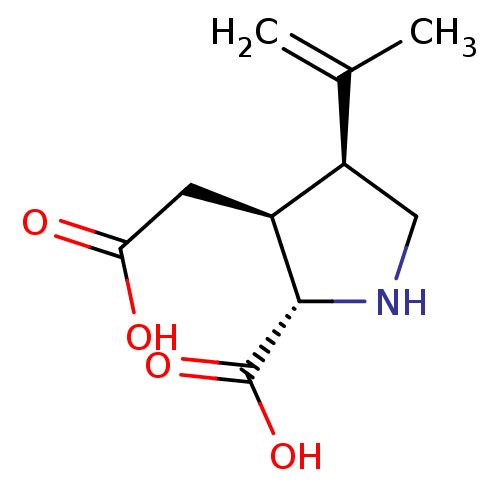 ((2S-(2alpha,3beta,4beta))-2-carboxy-4-(1-methyleth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Inhibitory concentration against human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50399433 (CHEMBL2178917) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 164 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrophysiology ... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50399432 (CHEMBL2178918) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen Curated by ChEMBL | Assay Description Antagonist activity at rat GluK1 receptor expressed in Xenopus oocytes at membrane potential -60 mV by two-electrode voltage-clamp electrophysiology ... | J Med Chem 55: 10297-301 (2012) Article DOI: 10.1021/jm301255m BindingDB Entry DOI: 10.7270/Q27S7PXR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50234094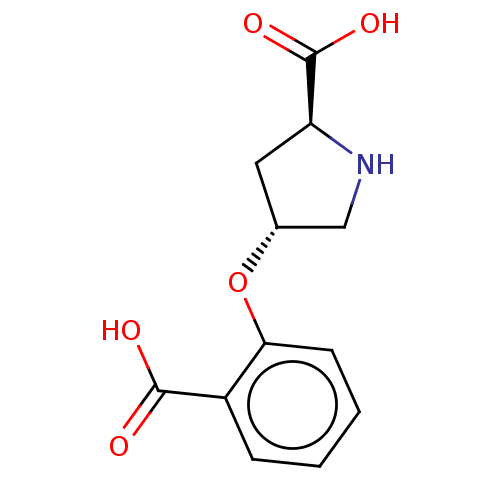 (CHEMBL4089715) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University Curated by ChEMBL | Assay Description Antagonist activity at recombinant rat full length GluK1 receptor expressed in HEK293T cells assessed as inhibition of glutamate induced increase in ... | J Med Chem 60: 441-457 (2017) Article DOI: 10.1021/acs.jmedchem.6b01516 BindingDB Entry DOI: 10.7270/Q2G44SJJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50175229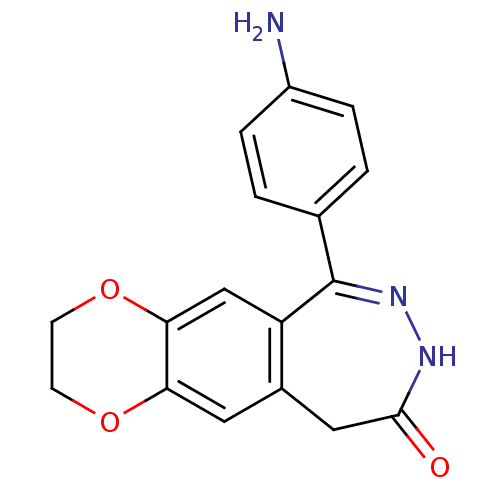 (6-(4-amino-phenyl)-2,3,8,10-tetrahydro-1,4-dioxa-7...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Università di Messina Curated by ChEMBL | Assay Description Ability to inhibit domoic acid-induced increase of calcium in rat HEK293 cells expressing GluR5 | Bioorg Med Chem Lett 16: 167-70 (2005) Article DOI: 10.1016/j.bmcl.2005.09.029 BindingDB Entry DOI: 10.7270/Q2P84BF2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109653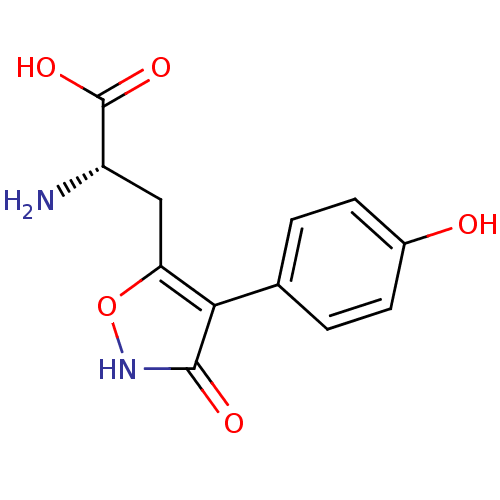 (2-Amino-3-[3-hydroxy-4-(4-hydroxy-phenyl)-isoxazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109654 (2-Amino-3-(3-hydroxy-4-pyridin-2-yl-isoxazol-5-yl)...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109658 (2-Amino-3-(3-hydroxy-4-phenethyl-isoxazol-5-yl)-pr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50089901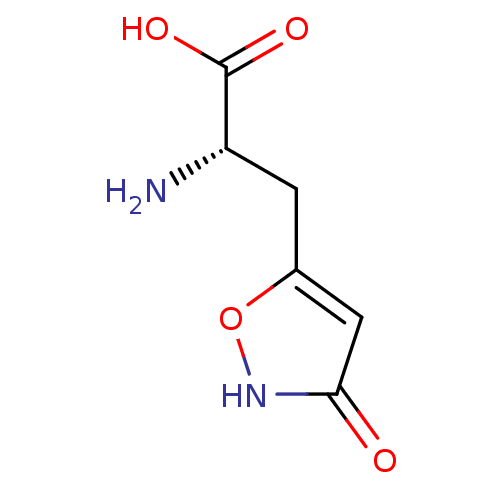 (2-Amino-3-(3-hydroxy-isoxazol-5-yl)-propionic acid...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109652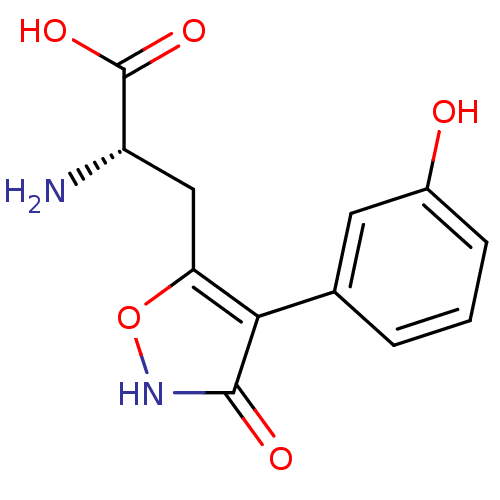 (2-Amino-3-[3-hydroxy-4-(3-hydroxy-phenyl)-isoxazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109656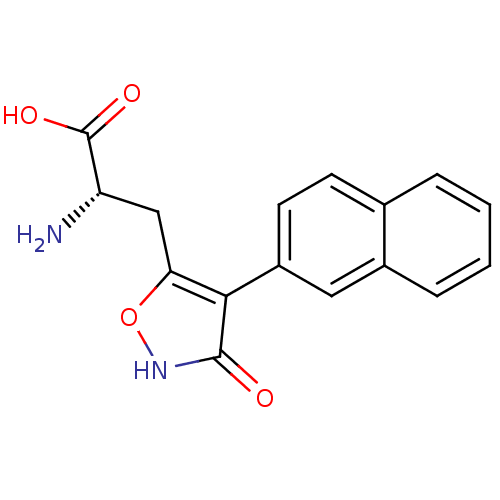 (2-Amino-3-(3-hydroxy-4-naphthalen-2-yl-isoxazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109657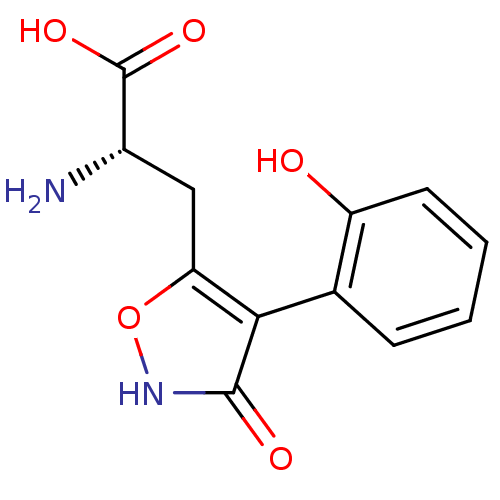 (2-Amino-3-[3-hydroxy-4-(2-hydroxy-phenyl)-isoxazol...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50109655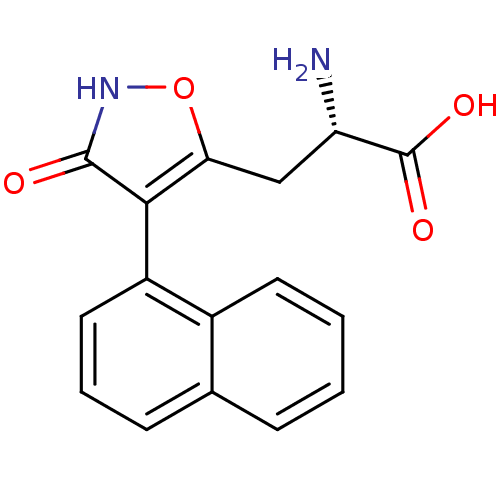 (2-Amino-3-(3-hydroxy-4-naphthalen-1-yl-isoxazol-5-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, kainate 1 (RAT) | BDBM50098248 (2-Amino-3-(3-hydroxy-4-phenyl-isoxazol-5-yl)-propi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Royal Danish School of Pharmacy Curated by ChEMBL | Assay Description Concentration of the compound which causes 50% reduction in receptor binding affinity against Ionotropic glutamate receptor ionotropic kainate using ... | J Med Chem 45: 988-91 (2002) BindingDB Entry DOI: 10.7270/Q2M32V27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||