

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50446676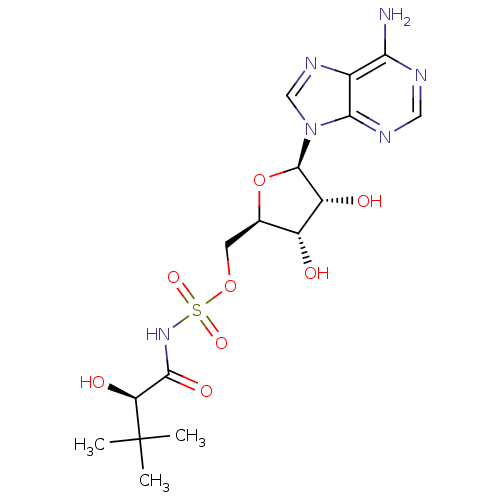 (CHEMBL1230349) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis recombinant PanC expressed in Escherichia coli BL21 (DE3) using pantoic acid and ATP as substrat... | Bioorg Med Chem 22: 1726-35 (2014) Article DOI: 10.1016/j.bmc.2014.01.017 BindingDB Entry DOI: 10.7270/Q2Q81FJT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50400279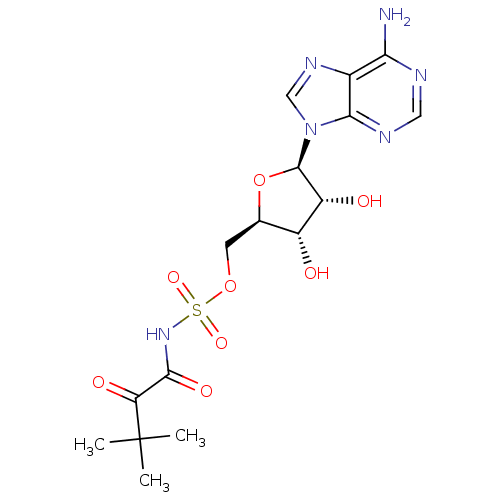 (CHEMBL2181057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis Pantothenate synthetase | J Med Chem 54: 915-29 (2011) Article DOI: 10.1021/jm101121s BindingDB Entry DOI: 10.7270/Q2NS0W23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50400282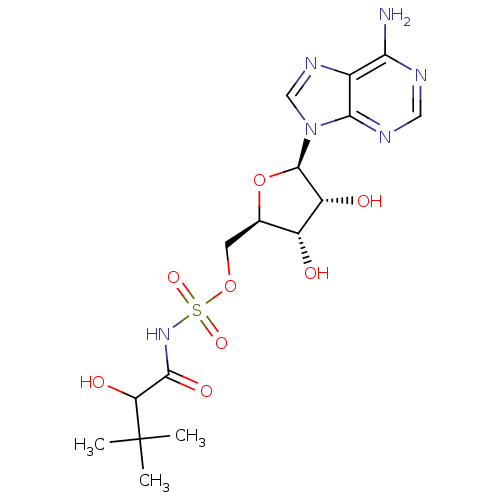 (CHEMBL2181060) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis Pantothenate synthetase | J Med Chem 54: 915-29 (2011) Article DOI: 10.1021/jm101121s BindingDB Entry DOI: 10.7270/Q2NS0W23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50400281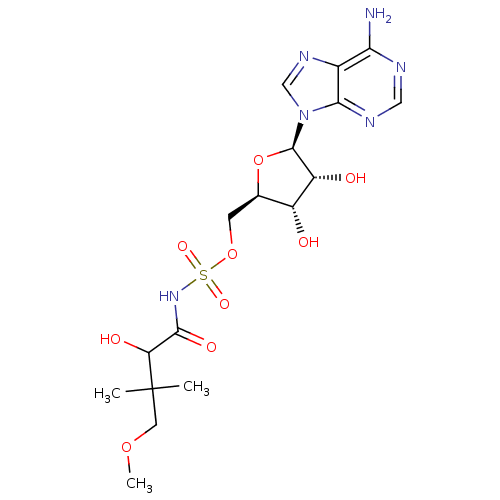 (CHEMBL2181059) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis Pantothenate synthetase | J Med Chem 54: 915-29 (2011) Article DOI: 10.1021/jm101121s BindingDB Entry DOI: 10.7270/Q2NS0W23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50400280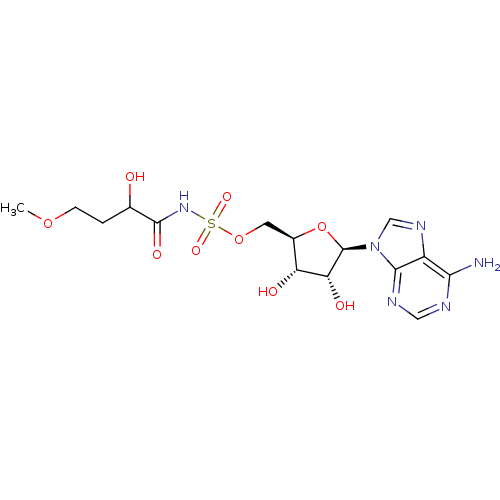 (CHEMBL2181058) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis Pantothenate synthetase | J Med Chem 54: 915-29 (2011) Article DOI: 10.1021/jm101121s BindingDB Entry DOI: 10.7270/Q2NS0W23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50400283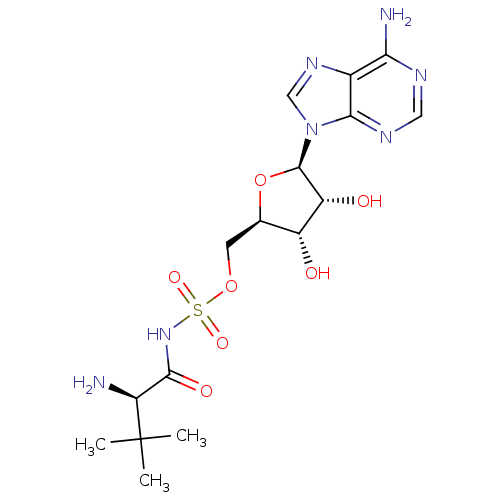 (CHEMBL1230355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ljubljana Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis Pantothenate synthetase | J Med Chem 54: 915-29 (2011) Article DOI: 10.1021/jm101121s BindingDB Entry DOI: 10.7270/Q2NS0W23 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50446673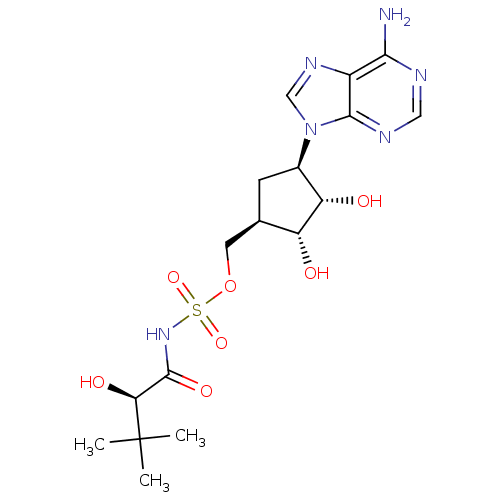 (CHEMBL3113591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis recombinant PanC expressed in Escherichia coli BL21 (DE3) using pantoic acid and ATP as substrat... | Bioorg Med Chem 22: 1726-35 (2014) Article DOI: 10.1016/j.bmc.2014.01.017 BindingDB Entry DOI: 10.7270/Q2Q81FJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50446674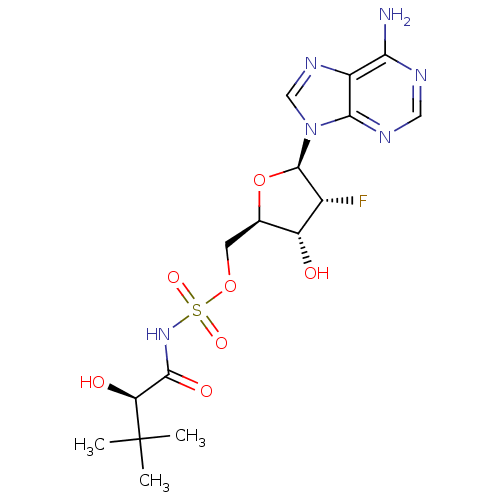 (CHEMBL3113590) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis recombinant PanC expressed in Escherichia coli BL21 (DE3) using pantoic acid and ATP as substrat... | Bioorg Med Chem 22: 1726-35 (2014) Article DOI: 10.1016/j.bmc.2014.01.017 BindingDB Entry DOI: 10.7270/Q2Q81FJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50347134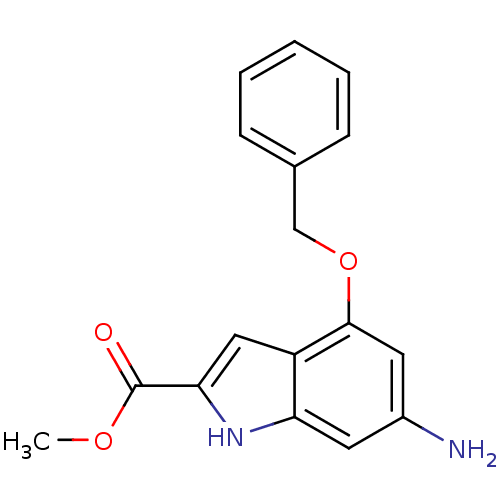 (CHEMBL1797249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis pantothenate synthetase using ATP by Dixon plot analysis | Bioorg Med Chem Lett 21: 3943-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.021 BindingDB Entry DOI: 10.7270/Q2X63N93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50446675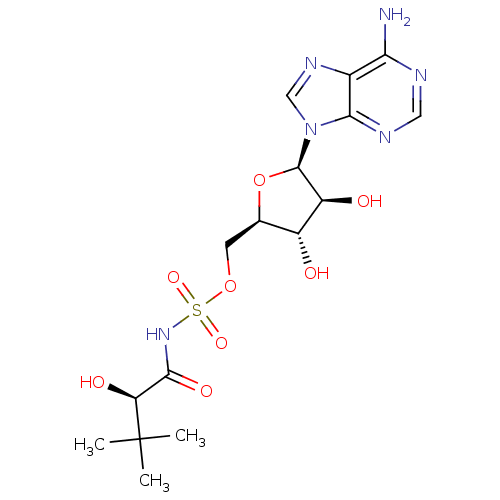 (CHEMBL3113589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis recombinant PanC expressed in Escherichia coli BL21 (DE3) using pantoic acid and ATP as substrat... | Bioorg Med Chem 22: 1726-35 (2014) Article DOI: 10.1016/j.bmc.2014.01.017 BindingDB Entry DOI: 10.7270/Q2Q81FJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50446672 (CHEMBL3113592) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Soochow University Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis recombinant PanC expressed in Escherichia coli BL21 (DE3) using pantoic acid and ATP as substrat... | Bioorg Med Chem 22: 1726-35 (2014) Article DOI: 10.1016/j.bmc.2014.01.017 BindingDB Entry DOI: 10.7270/Q2Q81FJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50347134 (CHEMBL1797249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis pantothenate synthetase using beta-alanine by Dixon plot analysis | Bioorg Med Chem Lett 21: 3943-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.021 BindingDB Entry DOI: 10.7270/Q2X63N93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||