

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043763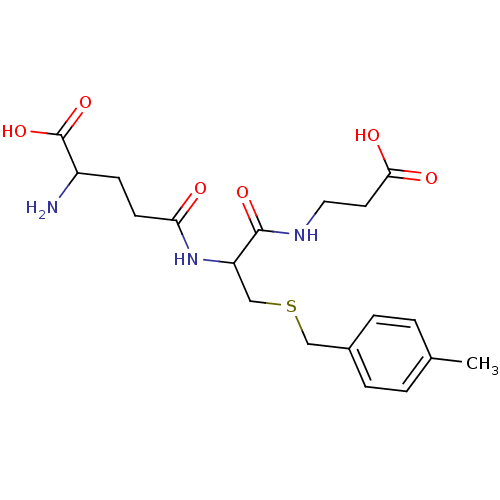 (2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-(4-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043759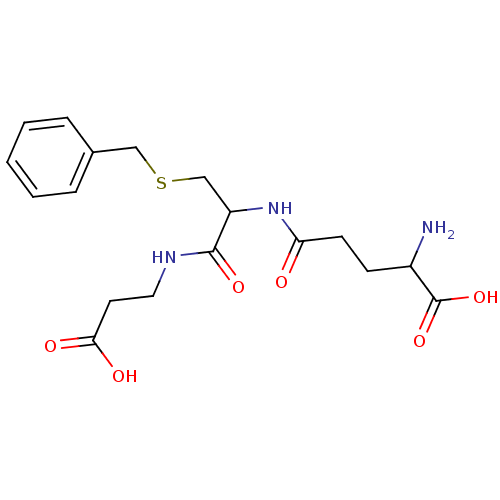 (2-Amino-4-[2-benzylsulfanyl-1-(2-carboxy-ethylcarb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043758 (2-Amino-4-[1-(carboxymethyl-carbamoyl)-2-hexylsulf...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043761 (2-Amino-4-[1-(2-carboxy-ethylcarbamoyl)-2-hexylsul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043760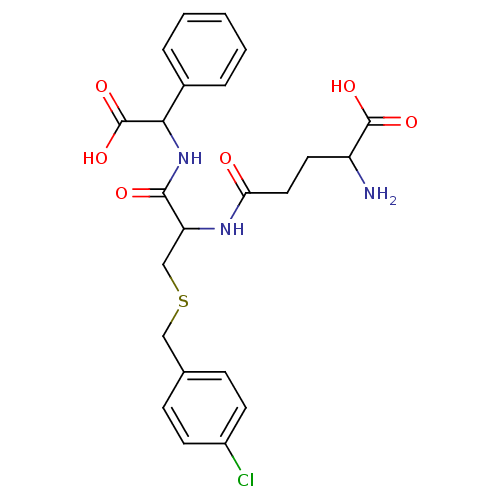 (2-Amino-4-[1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043764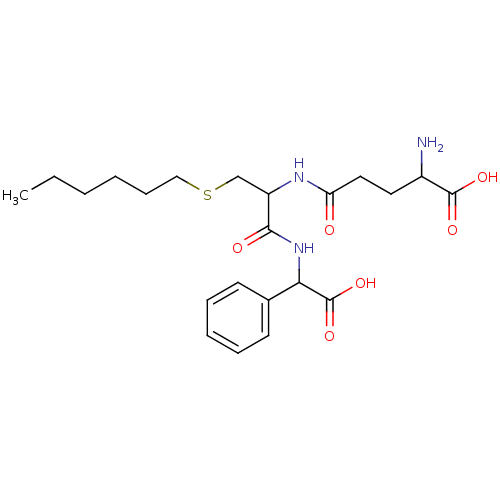 (2-Amino-4-{1-[(carboxy-phenyl-methyl)-carbamoyl]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutathione S-transferase Mu 2 (Homo sapiens (Human)) | BDBM50043762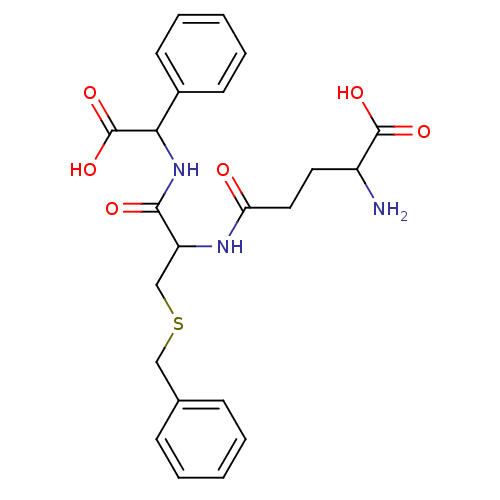 (2-Amino-4-{2-benzylsulfanyl-1-[(carboxy-phenyl-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PubMed | 1.84E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Terrapin Technologies Curated by ChEMBL | Assay Description Inhibitory activity of the compound was measured on recombinant human Glutathione S-transferase Mu 2 | J Med Chem 37: 189-94 (1994) BindingDB Entry DOI: 10.7270/Q21V5D1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||