

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50064254 ((2E,4E,6E,8Z)-3,7-Dimethyl-9-(2,6,6-trimethyl-cycl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 27 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50032675 (4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Transactivation of Gal4-LBD fused mouse RXRalpha (218 to 467) transfected in african green monkey CV1 cells assessed as luciferase activity at after ... | Eur J Med Chem 44: 2434-46 (2009) Article DOI: 10.1016/j.ejmech.2009.01.011 BindingDB Entry DOI: 10.7270/Q26Q1X99 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50082042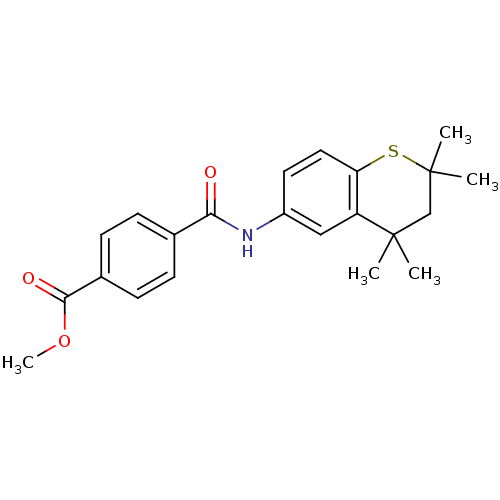 (CHEMBL139536 | N-(2,2,4,4-Tetramethyl-thiochroman-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 84 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation of CV-1 cells expressing murine Retinoid X receptor RXR alpha | J Med Chem 42: 4434-45 (1999) BindingDB Entry DOI: 10.7270/Q2891530 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50064253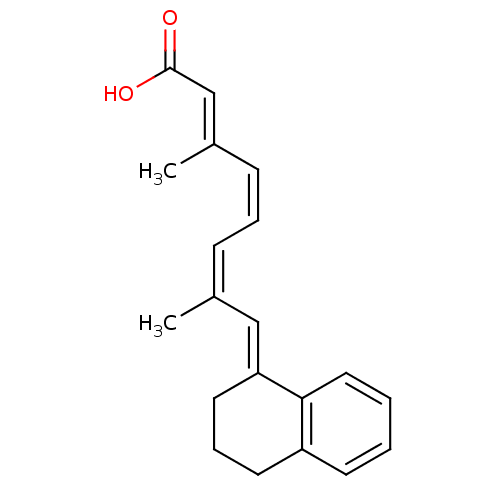 ((2E,4Z,6Z)-8-[3,4-Dihydro-2H-naphthalen-(1E)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 118 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM31892 (9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Transactivation of Gal4-LBD fused mouse RXRalpha (218 to 467) transfected in african green monkey CV1 cells assessed as luciferase activity at after ... | Eur J Med Chem 44: 2434-46 (2009) Article DOI: 10.1016/j.ejmech.2009.01.011 BindingDB Entry DOI: 10.7270/Q26Q1X99 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50064255 ((2E,4Z,6Z)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 220 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50082041 (4-iminomethylphenyl 4,4-dimethyl-6-chromanecarboxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 294 | n/a | n/a | n/a | n/a |
University of Oklahoma Health Sciences Center Curated by ChEMBL | Assay Description Transcriptional activation of CV-1 cells expressing murine Retinoid X receptor RXR alpha | J Med Chem 42: 4434-45 (1999) BindingDB Entry DOI: 10.7270/Q2891530 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50293410 ((E)-3-[5'-(Adamant-1-yl)-2-chloro-4'-hydroxy-2'-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 576 | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Transactivation of Gal4-LBD fused mouse RXRalpha (218 to 467) transfected in african green monkey CV1 cells assessed as luciferase activity at after ... | Eur J Med Chem 44: 2434-46 (2009) Article DOI: 10.1016/j.ejmech.2009.01.011 BindingDB Entry DOI: 10.7270/Q26Q1X99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50293413 ((E)-3-[5'-(Adamant-1-yl)-2-chloro-4'-[(2-methoxyet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 600 | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Transactivation of Gal4-LBD fused mouse RXRalpha (218 to 467) transfected in african green monkey CV1 cells assessed as luciferase activity at after ... | Eur J Med Chem 44: 2434-46 (2009) Article DOI: 10.1016/j.ejmech.2009.01.011 BindingDB Entry DOI: 10.7270/Q26Q1X99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50293412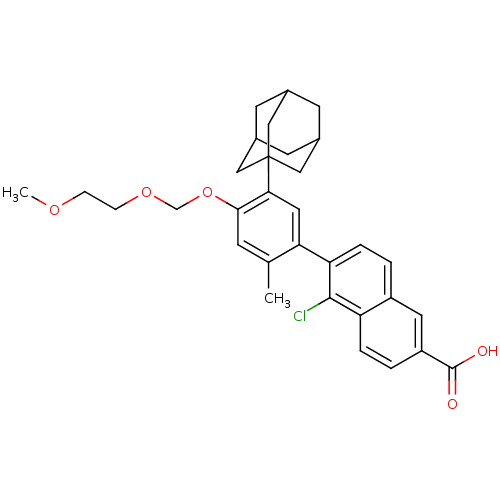 (6-[5-(Adamant-1-yl)-4-[(2-methoxyethoxy)methoxy]-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 667 | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Transactivation of Gal4-LBD fused mouse RXRalpha (218 to 467) transfected in african green monkey CV1 cells assessed as luciferase activity at after ... | Eur J Med Chem 44: 2434-46 (2009) Article DOI: 10.1016/j.ejmech.2009.01.011 BindingDB Entry DOI: 10.7270/Q26Q1X99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50293411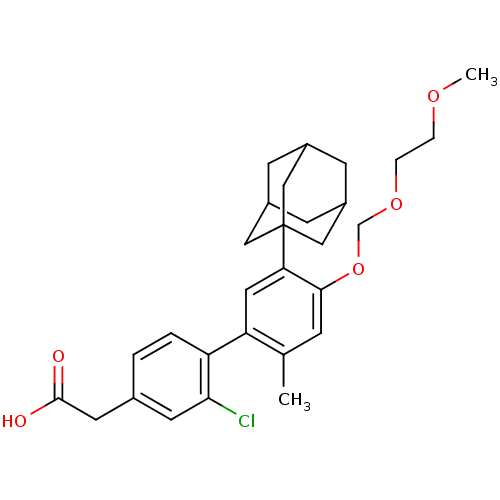 (2-[5'-(Adamant-1-yl)-2-chloro-4'-[(2-methoxyethoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a |
Universidade de Vigo Curated by ChEMBL | Assay Description Transactivation of Gal4-LBD fused mouse RXRalpha (218 to 467) transfected in african green monkey CV1 cells assessed as luciferase activity at after ... | Eur J Med Chem 44: 2434-46 (2009) Article DOI: 10.1016/j.ejmech.2009.01.011 BindingDB Entry DOI: 10.7270/Q26Q1X99 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50064250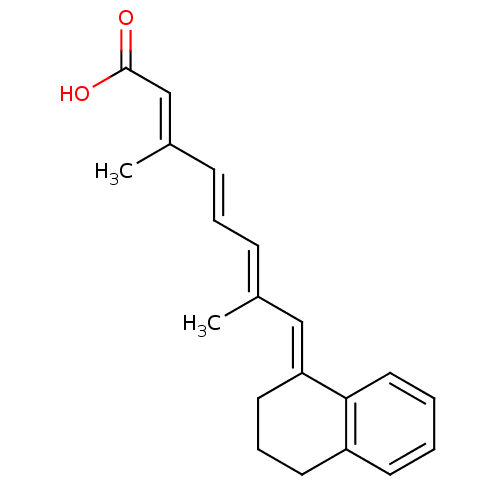 ((2E,4E,6E)-8-[3,4-Dihydro-2H-naphthalen-(1E)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM31883 (9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50064252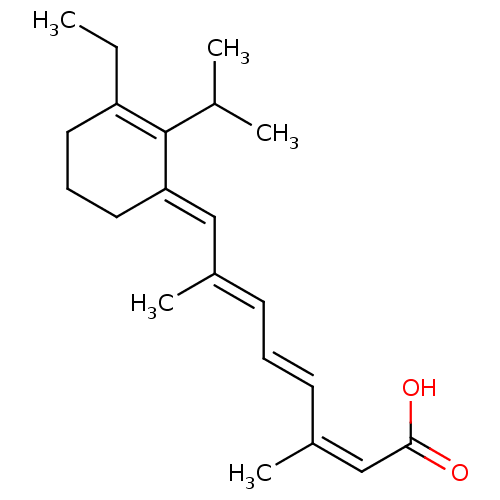 ((2Z,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50064251 ((2Z,4E,6E)-8-[3,4-Dihydro-2H-naphthalen-(1E)-ylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Mus musculus) | BDBM50031460 ((2E,4E,6E)-8-[3-Ethyl-2-isopropyl-cyclohex-2-en-(E...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a |
University of Alabama at Birmingham Curated by ChEMBL | Assay Description Transcriptional activation in CV-1 cells expressing retinoid X receptor RXR alpha | J Med Chem 41: 1679-87 (1998) Article DOI: 10.1021/jm970635h BindingDB Entry DOI: 10.7270/Q20864F4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||