 Found 7 hits of ic50 for UniProtKB: P17405
Found 7 hits of ic50 for UniProtKB: P17405 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50214969

(1,3,6-Trihydroxy-7-methoxy-2,8-bis-(3-methyl-but-2...)Show SMILES [#6]-[#8]-c1c(-[#8])cc2oc3cc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c3c(=O)c2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C24H26O6/c1-12(2)6-8-14-16(25)10-19-21(22(14)27)23(28)20-15(9-7-13(3)4)24(29-5)17(26)11-18(20)30-19/h6-7,10-11,25-27H,8-9H2,1-5H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.26E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acid sphingomyelinase |
Bioorg Med Chem Lett 13: 3151-3 (2003)
BindingDB Entry DOI: 10.7270/Q2R49SZ9 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50221662
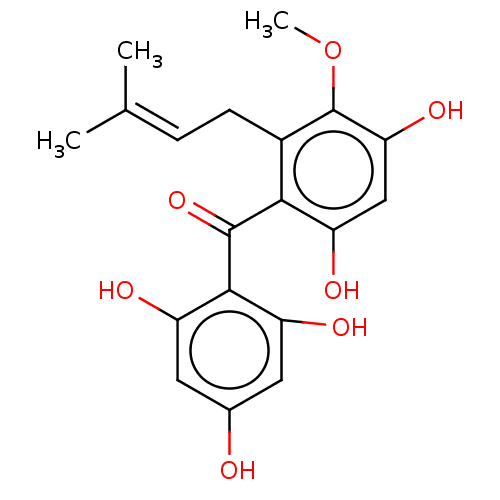
(CHEMBL320658)Show SMILES [#6]-[#8]-c1c(-[#8])cc(-[#8])c(-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8])c1-[#6]\[#6]=[#6](/[#6])-[#6] Show InChI InChI=1S/C19H20O7/c1-9(2)4-5-11-16(14(23)8-15(24)19(11)26-3)18(25)17-12(21)6-10(20)7-13(17)22/h4,6-8,20-24H,5H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acid sphingomyelinase |
Bioorg Med Chem Lett 13: 3151-3 (2003)
BindingDB Entry DOI: 10.7270/Q2R49SZ9 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50221658

(CHEMBL110491)Show SMILES [#6]-[#8]-c1c(-[#8])cc2oc3cc(-[#8])cc(-[#8])c3c(=O)c2c1-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C19H18O6/c1-9(2)4-5-11-16-15(8-13(22)19(11)24-3)25-14-7-10(20)6-12(21)17(14)18(16)23/h4,6-8,20-22H,5H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acid sphingomyelinase |
Bioorg Med Chem Lett 13: 3151-3 (2003)
BindingDB Entry DOI: 10.7270/Q2R49SZ9 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50221661
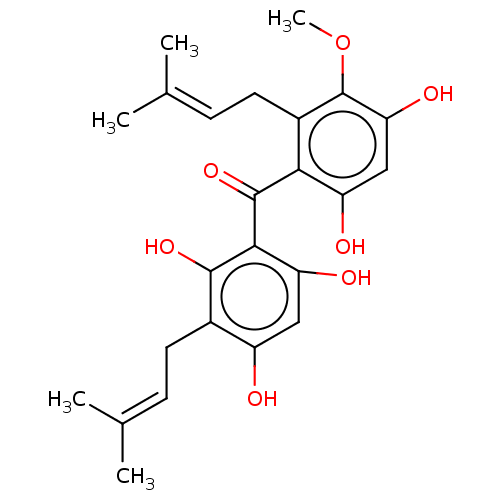
(CHEMBL110445)Show SMILES [#6]-[#8]-c1c(-[#8])cc(-[#8])c(-[#6](=O)-c2c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c2-[#8])c1-[#6]\[#6]=[#6](/[#6])-[#6] Show InChI InChI=1S/C24H28O7/c1-12(2)6-8-14-16(25)10-18(27)21(22(14)29)23(30)20-15(9-7-13(3)4)24(31-5)19(28)11-17(20)26/h6-7,10-11,25-29H,8-9H2,1-5H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acid sphingomyelinase |
Bioorg Med Chem Lett 13: 3151-3 (2003)
BindingDB Entry DOI: 10.7270/Q2R49SZ9 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50122309
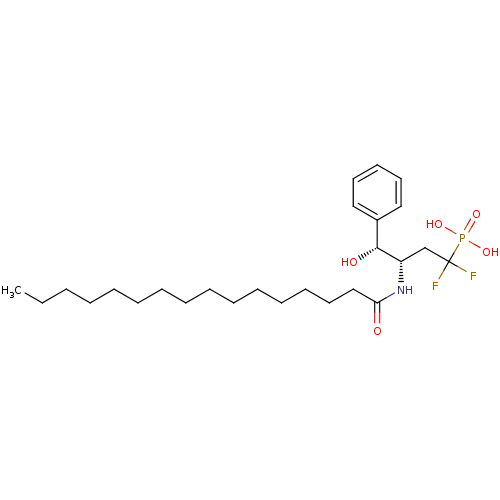
(((3S,4R)-1,1-Difluoro-3-hexadecanoylamino-4-hydrox...)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CC(F)(F)P(O)(O)=O)[C@H](O)c1ccccc1 Show InChI InChI=1S/C26H44F2NO5P/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-24(30)29-23(21-26(27,28)35(32,33)34)25(31)22-18-15-14-16-19-22/h14-16,18-19,23,25,31H,2-13,17,20-21H2,1H3,(H,29,30)(H2,32,33,34)/t23-,25+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo University of Pharmacy & Life Science
Curated by ChEMBL
| Assay Description
Inhibitory activity of the compound against Acid sphingomyelinase from bovine brain microsome |
Bioorg Med Chem Lett 13: 229-36 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HFH |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50221660

(CHEMBL109045)Show SMILES [#6]-[#8]-c1cc(-[#6](=O)-c2c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](/[#6])-[#6])c2-[#8])c(-[#8])cc1-[#8] Show InChI InChI=1S/C19H20O7/c1-9(2)4-5-10-12(20)8-15(23)17(18(10)24)19(25)11-6-16(26-3)14(22)7-13(11)21/h4,6-8,20-24H,5H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acid sphingomyelinase |
Bioorg Med Chem Lett 13: 3151-3 (2003)
BindingDB Entry DOI: 10.7270/Q2R49SZ9 |
More data for this
Ligand-Target Pair | |
Sphingomyelin phosphodiesterase [1-45,48-631]
(Homo sapiens (Human)) | BDBM50221659
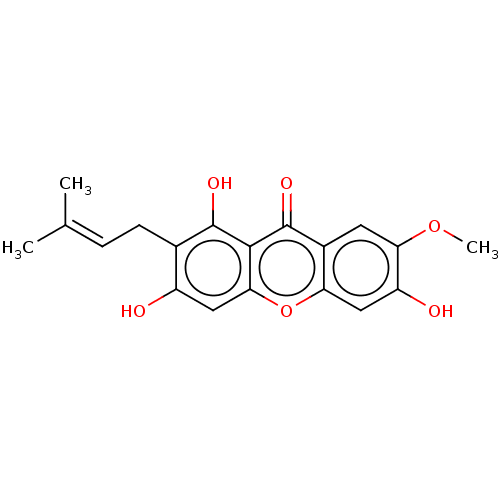
(CHEMBL107844)Show SMILES [#6]-[#8]-c1cc2c(cc1-[#8])oc1cc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])c1c2=O Show InChI InChI=1S/C19H18O6/c1-9(2)4-5-10-12(20)7-16-17(18(10)22)19(23)11-6-15(24-3)13(21)8-14(11)25-16/h4,6-8,20-22H,5H2,1-3H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Keio University
Curated by ChEMBL
| Assay Description
Inhibitory activity against Acid sphingomyelinase |
Bioorg Med Chem Lett 13: 3151-3 (2003)
BindingDB Entry DOI: 10.7270/Q2R49SZ9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data