

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277140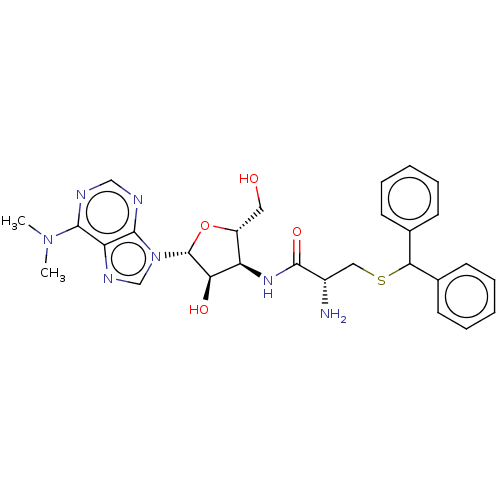 (CHEMBL3244800) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277143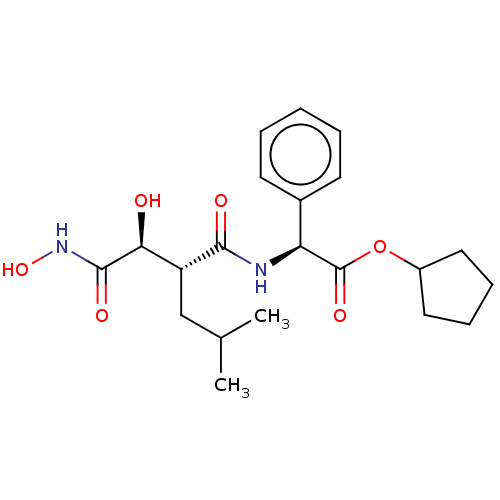 (CHR 2797 | CHR-2797 | Tosedostat) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277160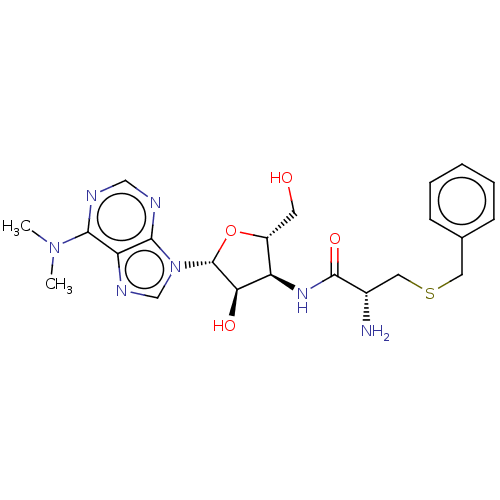 (CHEMBL3244799) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 940 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277156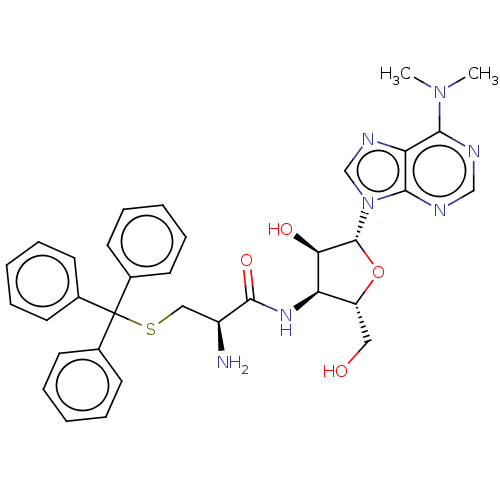 (CHEMBL4165328) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499710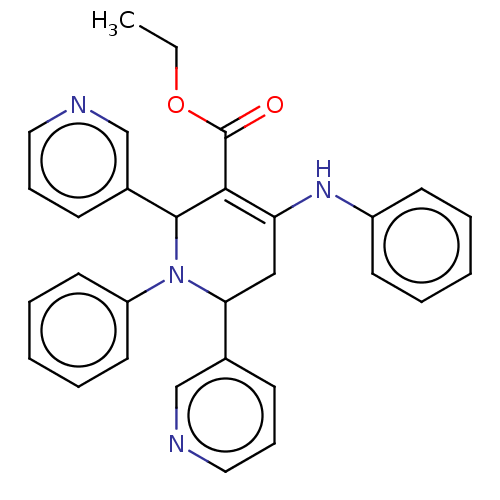 (CHEMBL516021) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277158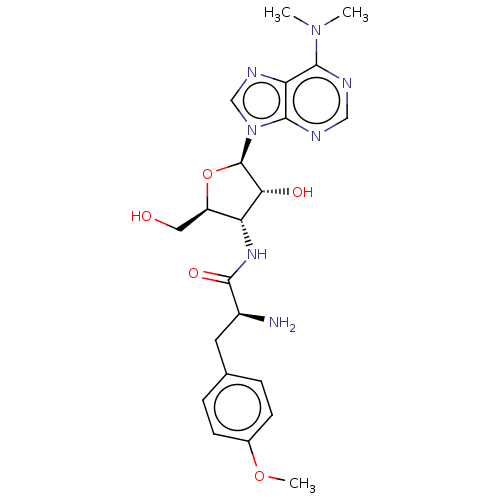 (3123L | CHEBI:17939 | CL-13900 | GNF-Pf-2016 | P-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50121712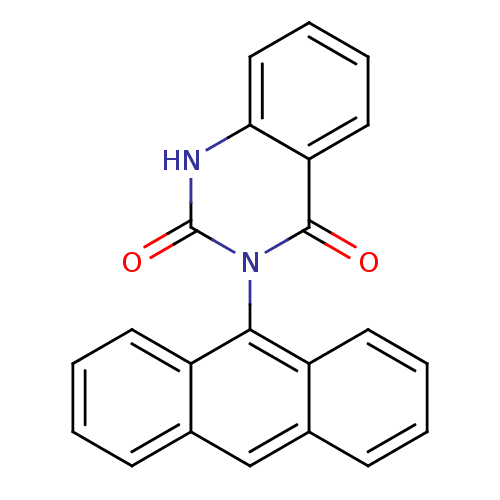 (ANTAQ | CHEMBL169736) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Puromycin sensitive aminopeptidase inhibitory activity of the compound was determined by the use of L-Ala AMC with MOLT-4 | Bioorg Med Chem Lett 13: 83-6 (2002) BindingDB Entry DOI: 10.7270/Q2RV0N1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50121708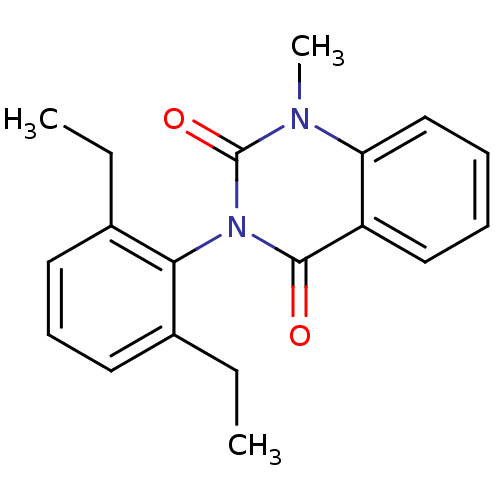 (CHEMBL168510 | MPAQ-22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Puromycin sensitive aminopeptidase inhibitory activity of the compound was determined by the use of L-Ala AMC with MOLT-4 | Bioorg Med Chem Lett 13: 83-6 (2002) BindingDB Entry DOI: 10.7270/Q2RV0N1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM23971 ((2S)-2-[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50121709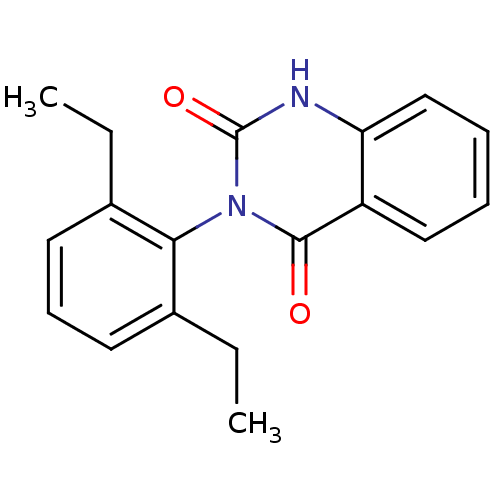 (CHEMBL169458 | PAQ-22) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Puromycin sensitive aminopeptidase inhibitory activity of the compound was determined by the use of L-Ala AMC with MOLT-4 | Bioorg Med Chem Lett 13: 83-6 (2002) BindingDB Entry DOI: 10.7270/Q2RV0N1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50121711 (CHEMBL169895 | DAMPAQ) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Puromycin sensitive aminopeptidase inhibitory activity of the compound was determined by the use of L-Ala AMC with MOLT-4 | Bioorg Med Chem Lett 13: 83-6 (2002) BindingDB Entry DOI: 10.7270/Q2RV0N1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277142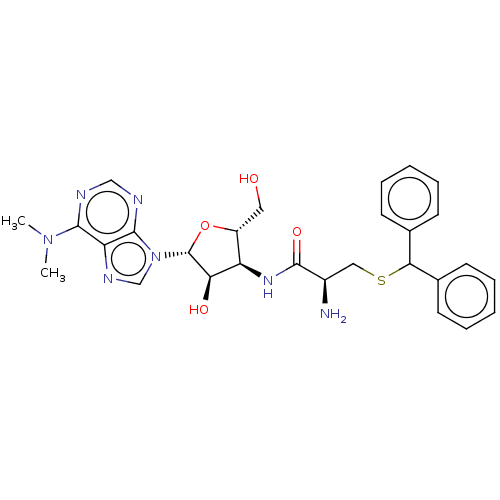 (CHEMBL4161892) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277141 (CHEMBL4161496) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50121710 (2-(2,6-Diethyl-phenyl)-4H-isoquinoline-1,3-dione |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Puromycin sensitive aminopeptidase inhibitory activity of the compound was determined by the use of L-Ala AMC with MOLT-4 | Bioorg Med Chem Lett 13: 83-6 (2002) BindingDB Entry DOI: 10.7270/Q2RV0N1C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499702 (CHEMBL3740370) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499709 (CHEMBL3741560) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277158 (3123L | CHEBI:17939 | CL-13900 | GNF-Pf-2016 | P-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499705 (CHEMBL3741724) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499712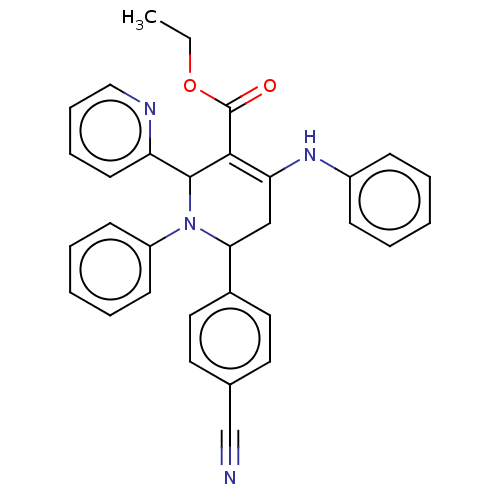 (CHEMBL3741833) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499707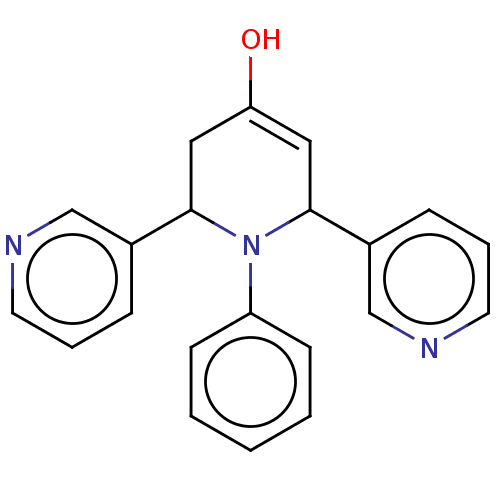 (CHEMBL3741904) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499708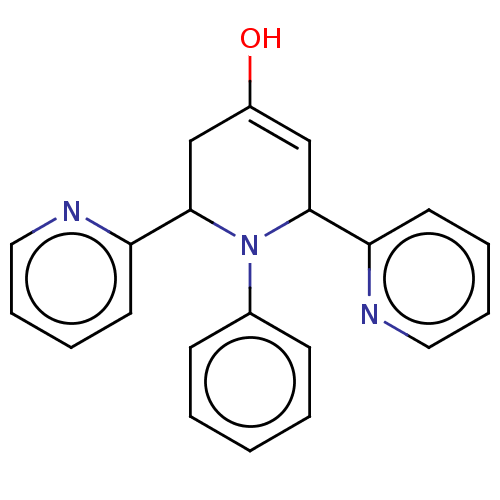 (CHEMBL3739649) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499706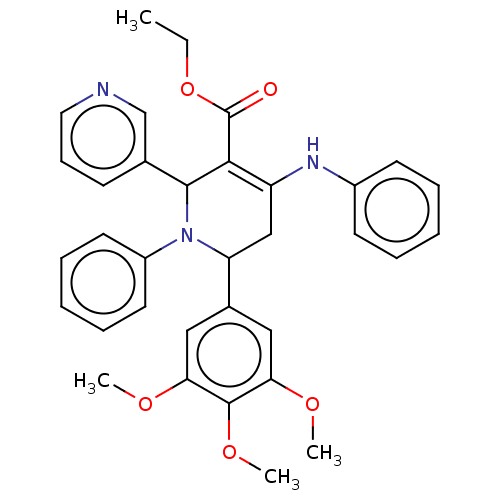 (CHEMBL3740199) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277157 (CHEMBL4173240) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277150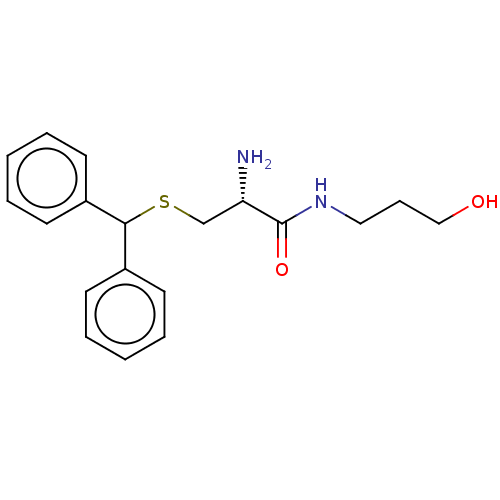 (CHEMBL4161744) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499704 (CHEMBL3740158) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499713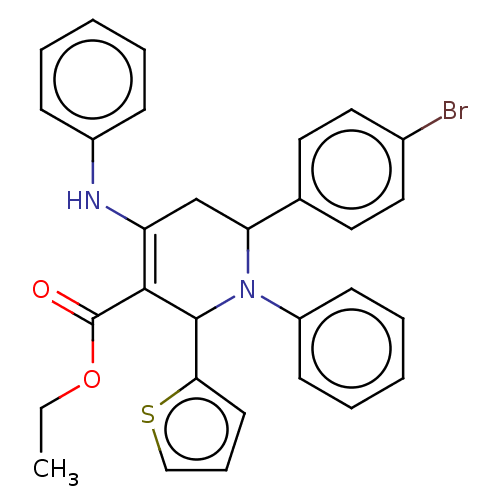 (CHEMBL3741344) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499701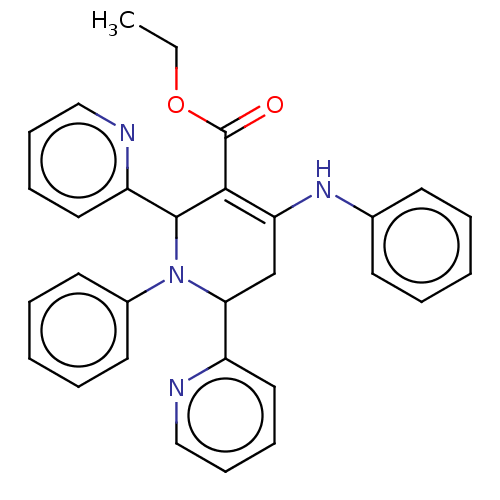 (CHEMBL3741701) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499703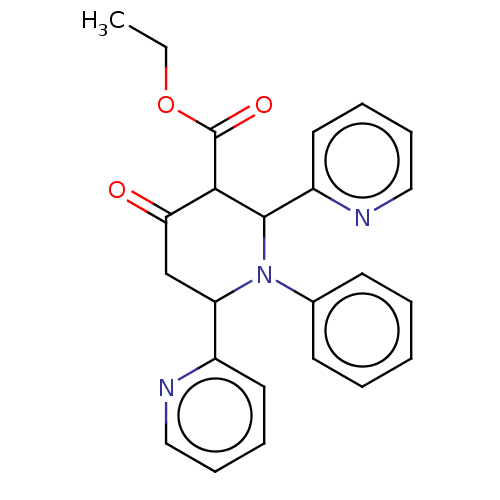 (CHEMBL3740862) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50499711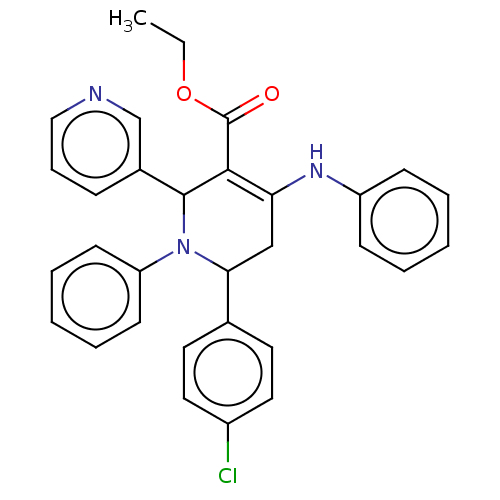 (CHEMBL3741419) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIR-Indian Institute of Chemical Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human puromycin sensitive aminopeptidase using Leu-pNA as substrate after 30 mins | Eur J Med Chem 106: 26-33 (2015) Article DOI: 10.1016/j.ejmech.2015.10.026 BindingDB Entry DOI: 10.7270/Q2FF3WCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Puromycin-sensitive aminopeptidase (Homo sapiens (Human)) | BDBM50277159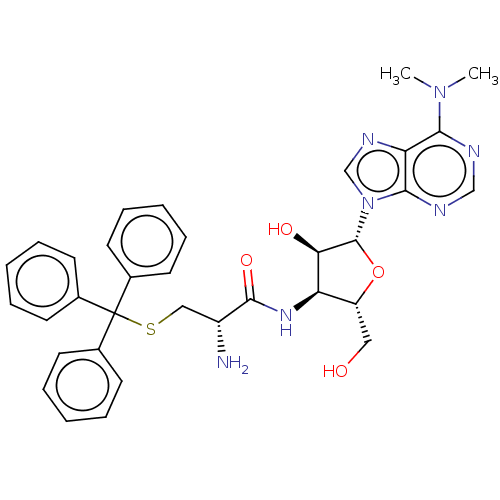 (CHEMBL4169814) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal His6-MBP-tagged PSA expressed in Escherichia coli BL21 STAR (DE3) using 4-Ala-MNA as substrate measured fo... | Eur J Med Chem 139: 325-336 (2017) Article DOI: 10.1016/j.ejmech.2017.07.048 BindingDB Entry DOI: 10.7270/Q2FT8PJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||