 Found 76 hits of ic50 for UniProtKB: P54687
Found 76 hits of ic50 for UniProtKB: P54687 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607315

(BAY-069)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)F)c2ccccc2c1Cl |(20.53,-12.27,;19.26,-11.42,;19.36,-9.88,;18.08,-9.03,;16.7,-9.71,;16.59,-11.25,;17.87,-12.1,;17.76,-13.64,;16.38,-14.32,;15.11,-13.47,;13.73,-14.15,;12.45,-13.3,;12.56,-11.76,;13.94,-11.08,;11.28,-10.9,;9.9,-11.59,;9.79,-13.13,;11.07,-13.98,;10.96,-15.52,;8.45,-11.09,;8.55,-9.56,;7.17,-10.24,;7.29,-12.11,;13.62,-15.7,;12.24,-16.38,;12.13,-17.91,;13.41,-18.77,;14.79,-18.09,;14.9,-16.55,;16.28,-15.87,;17.55,-16.72,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607303
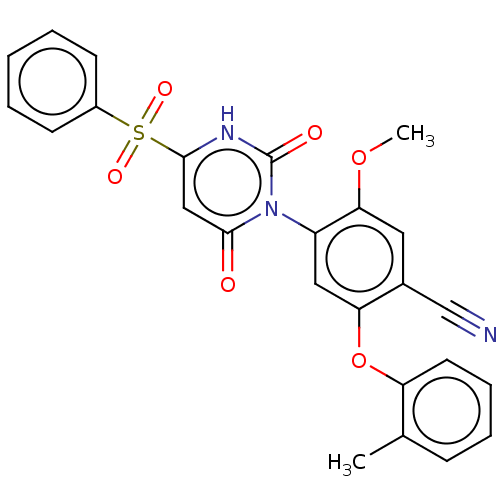
(CHEMBL5220763)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)S(=O)(=O)c1ccccc1 |(3.44,-2.7,;2.11,-1.93,;2.11,-.39,;3.44,.39,;3.44,1.92,;4.77,2.69,;6.1,3.46,;2.1,2.69,;2.1,4.23,;3.44,5,;4.78,4.23,;6.1,5.01,;6.1,6.54,;4.77,7.31,;3.44,6.54,;2.1,7.31,;.77,1.92,;.77,.38,;-.56,-.38,;-1.9,.39,;-1.9,1.92,;-3.23,-.38,;-3.23,-1.93,;-1.9,-2.7,;-.56,-1.93,;.77,-2.7,;-4.56,-2.7,;-6.1,-2.7,;-4.96,-1.21,;-4.56,-4.24,;-3.23,-5.01,;-3.23,-6.54,;-4.57,-7.31,;-5.89,-6.55,;-5.9,-5.01,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50118665

(CHEMBL3617085)Show SMILES CCCCc1cc(=O)n2nc(NCc3c(F)cc(Cl)cc3F)c(C#N)c2[nH]1 Show InChI InChI=1S/C18H16ClF2N5O/c1-2-3-4-11-7-16(27)26-18(24-11)12(8-22)17(25-26)23-9-13-14(20)5-10(19)6-15(13)21/h5-7,24H,2-4,9H2,1H3,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BCATc (unknown origin) |
J Med Chem 58: 7140-63 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00313
BindingDB Entry DOI: 10.7270/Q2T43VWK |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607314
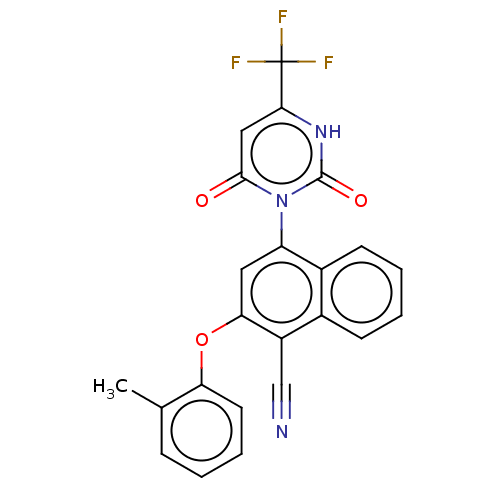
(CHEMBL5219349)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)F)c2ccccc2c1C#N |(2,5.77,;3.34,5,;4.67,5.77,;6,5,;6,3.47,;4.68,2.69,;3.34,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.66,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,;2.01,-1.93,;2.02,-3.47,;3.36,-4.23,;4.68,-3.45,;4.67,-1.91,;3.34,-1.15,;3.34,.38,;4.67,1.15,;6,1.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607314

(CHEMBL5219349)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)F)c2ccccc2c1C#N |(2,5.77,;3.34,5,;4.67,5.77,;6,5,;6,3.47,;4.68,2.69,;3.34,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.66,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,;2.01,-1.93,;2.02,-3.47,;3.36,-4.23,;4.68,-3.45,;4.67,-1.91,;3.34,-1.15,;3.34,.38,;4.67,1.15,;6,1.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607314

(CHEMBL5219349)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)F)c2ccccc2c1C#N |(2,5.77,;3.34,5,;4.67,5.77,;6,5,;6,3.47,;4.68,2.69,;3.34,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.66,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,;2.01,-1.93,;2.02,-3.47,;3.36,-4.23,;4.68,-3.45,;4.67,-1.91,;3.34,-1.15,;3.34,.38,;4.67,1.15,;6,1.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607311
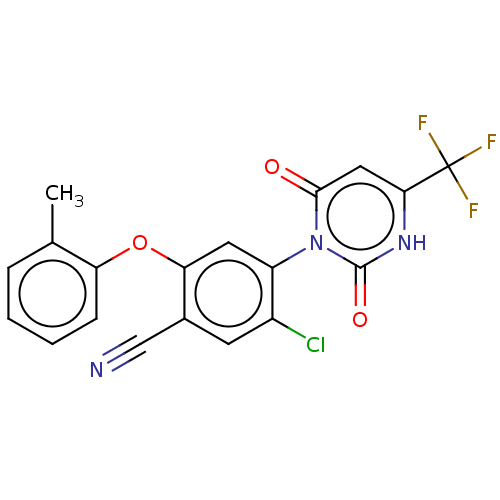
(CHEMBL5220953)Show SMILES Cc1ccccc1Oc1cc(c(Cl)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.34,5,;4.67,5.77,;6,5,;6,3.47,;4.68,2.69,;3.34,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.34,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.66,-4.23,;-4.66,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607300
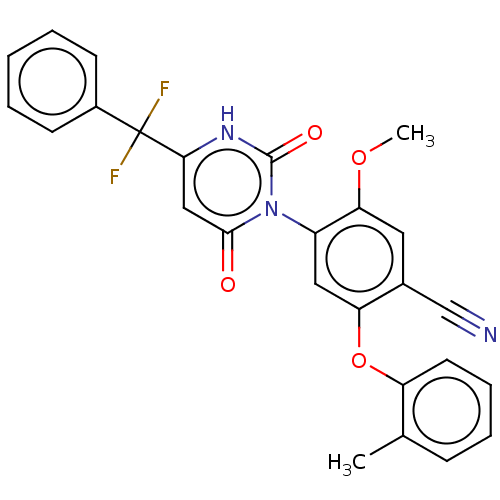
(CHEMBL5218588)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)c1ccccc1 |(3.42,-2.7,;2.08,-1.93,;2.08,-.39,;3.41,.39,;3.41,1.92,;4.74,2.69,;6.08,3.46,;2.08,2.69,;2.08,4.23,;3.41,5,;4.75,4.23,;6.08,5.01,;6.07,6.54,;4.74,7.31,;3.41,6.54,;2.08,7.31,;.75,1.92,;.75,.38,;-.59,-.39,;-1.92,.39,;-1.92,1.93,;-3.26,-.39,;-3.26,-1.93,;-1.92,-2.7,;-.59,-1.93,;.75,-2.7,;-4.59,-2.7,;-6.08,-3.09,;-5.36,-1.36,;-4.59,-4.24,;-3.26,-5.01,;-3.26,-6.54,;-4.59,-7.31,;-5.92,-6.55,;-5.93,-5.01,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607297
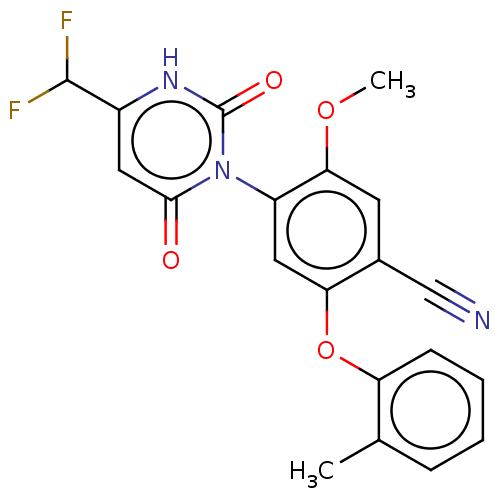
(CHEMBL5220319)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)F |(3.34,-4.24,;2.01,-3.47,;2.01,-1.93,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;2,1.15,;2,2.69,;3.33,3.46,;4.67,2.69,;6,3.47,;6,5,;4.66,5.77,;3.33,5,;2,5.77,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607286
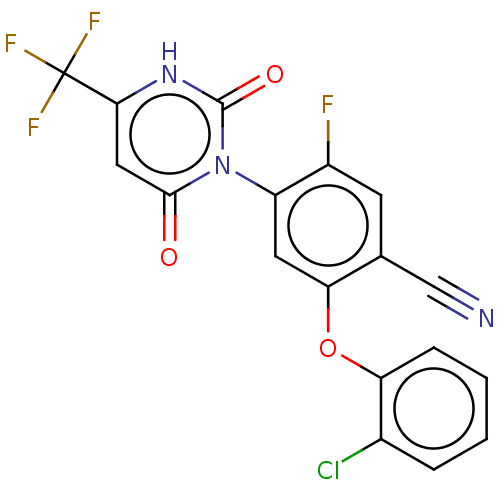
(CHEMBL5218805)Show SMILES Fc1cc(C#N)c(Oc2ccccc2Cl)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2.01,-3.47,;2.01,-1.93,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;2,1.15,;2,2.69,;3.33,3.46,;4.67,2.69,;6,3.47,;6,5,;4.66,5.77,;3.33,5,;2,5.77,;.67,.39,;.67,-1.15,;-.67,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.67,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607294

(CHEMBL5220178)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)F |(3.34,-4.24,;2.01,-3.47,;2.01,-1.93,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;2,1.15,;2,2.69,;3.33,3.46,;4.67,2.69,;6,3.47,;6,5,;4.66,5.77,;3.33,5,;2,5.77,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607316

(CHEMBL5219436)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)c2ccccc2)c2ccccc2c1Cl |(3.33,5.67,;4.67,4.9,;6,5.67,;7.33,4.9,;7.34,3.36,;6.01,2.59,;4.67,3.36,;3.33,2.59,;3.33,1.05,;2,.28,;2,-1.26,;.67,-2.03,;-.67,-1.26,;-.67,.28,;-2,-2.03,;-2,-3.57,;-.67,-4.34,;.67,-3.57,;2,-4.34,;-3.33,-4.34,;-4.1,-5.67,;-2.56,-5.67,;-4.67,-3.56,;-6.01,-4.33,;-7.34,-3.56,;-7.34,-2.02,;-6.01,-1.26,;-4.67,-2.02,;3.34,-2.03,;3.35,-3.58,;4.69,-4.33,;6.01,-3.55,;6,-2.01,;4.67,-1.25,;4.67,.28,;6,1.05,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607304
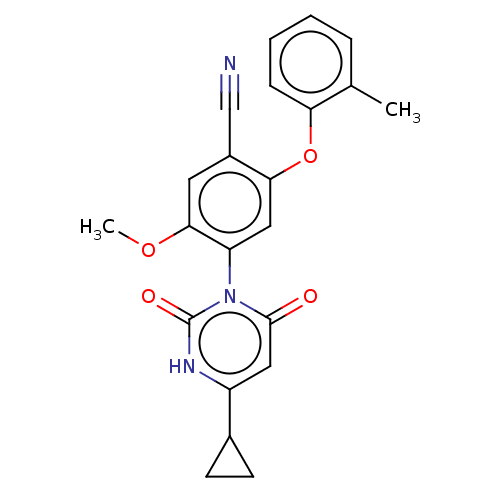
(CHEMBL5219314)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C1CC1 |(3.45,-4.34,;2.11,-3.57,;2.11,-2.03,;3.44,-1.25,;3.44,.28,;4.77,1.05,;6.11,1.82,;2.1,1.05,;2.1,2.59,;3.44,3.36,;4.78,2.59,;6.11,3.36,;6.1,4.9,;4.77,5.67,;3.44,4.9,;2.1,5.67,;.77,.28,;.77,-1.26,;-.56,-2.03,;-1.9,-1.26,;-1.9,.28,;-3.23,-2.03,;-3.23,-3.57,;-1.9,-4.34,;-.56,-3.57,;.77,-4.34,;-4.56,-4.34,;-6.11,-4.34,;-5.33,-5.67,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607315

(BAY-069)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)F)c2ccccc2c1Cl |(20.53,-12.27,;19.26,-11.42,;19.36,-9.88,;18.08,-9.03,;16.7,-9.71,;16.59,-11.25,;17.87,-12.1,;17.76,-13.64,;16.38,-14.32,;15.11,-13.47,;13.73,-14.15,;12.45,-13.3,;12.56,-11.76,;13.94,-11.08,;11.28,-10.9,;9.9,-11.59,;9.79,-13.13,;11.07,-13.98,;10.96,-15.52,;8.45,-11.09,;8.55,-9.56,;7.17,-10.24,;7.29,-12.11,;13.62,-15.7,;12.24,-16.38,;12.13,-17.91,;13.41,-18.77,;14.79,-18.09,;14.9,-16.55,;16.28,-15.87,;17.55,-16.72,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607315

(BAY-069)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)F)c2ccccc2c1Cl |(20.53,-12.27,;19.26,-11.42,;19.36,-9.88,;18.08,-9.03,;16.7,-9.71,;16.59,-11.25,;17.87,-12.1,;17.76,-13.64,;16.38,-14.32,;15.11,-13.47,;13.73,-14.15,;12.45,-13.3,;12.56,-11.76,;13.94,-11.08,;11.28,-10.9,;9.9,-11.59,;9.79,-13.13,;11.07,-13.98,;10.96,-15.52,;8.45,-11.09,;8.55,-9.56,;7.17,-10.24,;7.29,-12.11,;13.62,-15.7,;12.24,-16.38,;12.13,-17.91,;13.41,-18.77,;14.79,-18.09,;14.9,-16.55,;16.28,-15.87,;17.55,-16.72,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607316

(CHEMBL5219436)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)c2ccccc2)c2ccccc2c1Cl |(3.33,5.67,;4.67,4.9,;6,5.67,;7.33,4.9,;7.34,3.36,;6.01,2.59,;4.67,3.36,;3.33,2.59,;3.33,1.05,;2,.28,;2,-1.26,;.67,-2.03,;-.67,-1.26,;-.67,.28,;-2,-2.03,;-2,-3.57,;-.67,-4.34,;.67,-3.57,;2,-4.34,;-3.33,-4.34,;-4.1,-5.67,;-2.56,-5.67,;-4.67,-3.56,;-6.01,-4.33,;-7.34,-3.56,;-7.34,-2.02,;-6.01,-1.26,;-4.67,-2.02,;3.34,-2.03,;3.35,-3.58,;4.69,-4.33,;6.01,-3.55,;6,-2.01,;4.67,-1.25,;4.67,.28,;6,1.05,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607316

(CHEMBL5219436)Show SMILES Cc1ccccc1Oc1cc(-n2c(=O)cc([nH]c2=O)C(F)(F)c2ccccc2)c2ccccc2c1Cl |(3.33,5.67,;4.67,4.9,;6,5.67,;7.33,4.9,;7.34,3.36,;6.01,2.59,;4.67,3.36,;3.33,2.59,;3.33,1.05,;2,.28,;2,-1.26,;.67,-2.03,;-.67,-1.26,;-.67,.28,;-2,-2.03,;-2,-3.57,;-.67,-4.34,;.67,-3.57,;2,-4.34,;-3.33,-4.34,;-4.1,-5.67,;-2.56,-5.67,;-4.67,-3.56,;-6.01,-4.33,;-7.34,-3.56,;-7.34,-2.02,;-6.01,-1.26,;-4.67,-2.02,;3.34,-2.03,;3.35,-3.58,;4.69,-4.33,;6.01,-3.55,;6,-2.01,;4.67,-1.25,;4.67,.28,;6,1.05,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607301
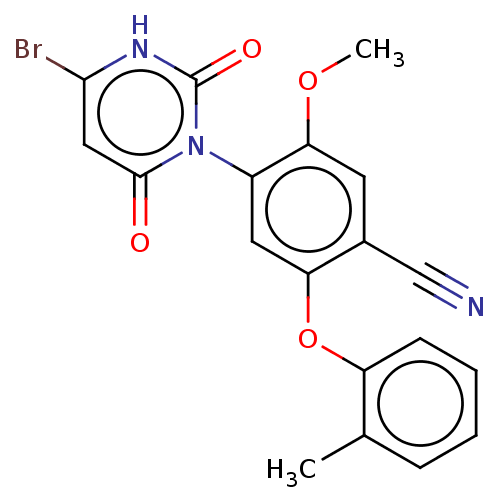
(CHEMBL5220805)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc(Br)[nH]c1=O |(2.67,-5,;1.34,-4.23,;1.34,-2.69,;2.67,-1.92,;2.67,-.39,;4,.38,;5.33,1.15,;1.33,.38,;1.33,1.92,;2.67,2.69,;4.01,1.92,;5.33,2.7,;5.33,4.23,;4,5,;2.67,4.24,;1.33,5,;0,-.38,;0,-1.92,;-1.33,-2.69,;-2.67,-1.92,;-2.67,-.38,;-4,-2.69,;-4,-4.23,;-5.33,-5,;-2.67,-5,;-1.33,-4.23,;0,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607298
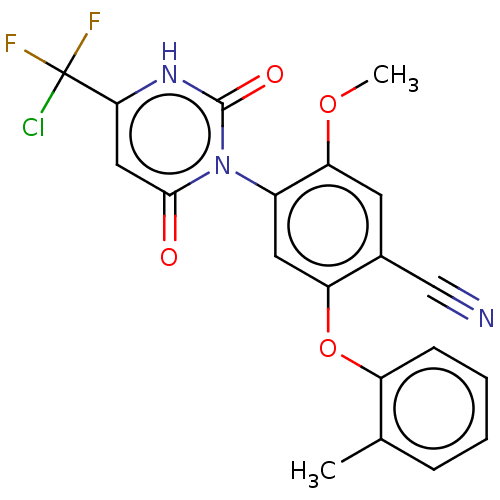
(CHEMBL5219261)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)Cl |(3.34,-4.24,;2.01,-3.47,;2.01,-1.93,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;2,1.15,;2,2.69,;3.33,3.46,;4.67,2.69,;6,3.47,;6,5,;4.66,5.77,;3.33,5,;2,5.77,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607291
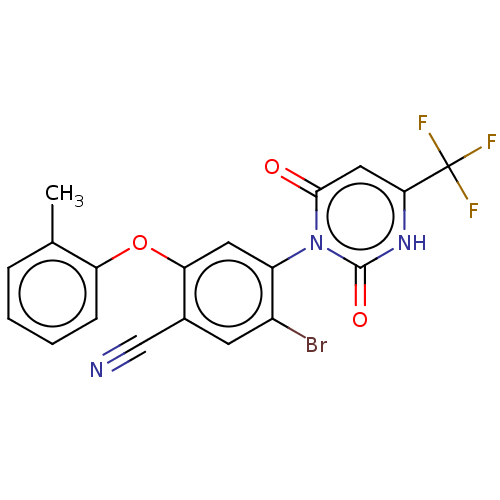
(CHEMBL5220903)Show SMILES Cc1ccccc1Oc1cc(c(Br)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.33,5,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;3.33,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50353005
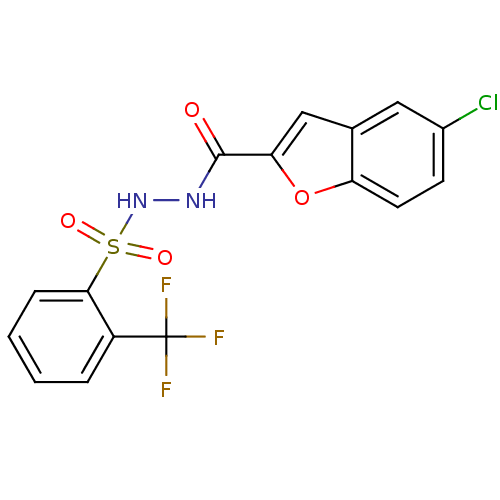
(CHEMBL1231666)Show SMILES FC(F)(F)c1ccccc1S(=O)(=O)NNC(=O)c1cc2cc(Cl)ccc2o1 Show InChI InChI=1S/C16H10ClF3N2O4S/c17-10-5-6-12-9(7-10)8-13(26-12)15(23)21-22-27(24,25)14-4-2-1-3-11(14)16(18,19)20/h1-8,22H,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant histidine-tagged BCATc expressed in Escherichia coli BL21 (DE3) after 50 mins using [13C]-L-leucine as substrate by N... |
Bioorg Med Chem Lett 21: 5248-50 (2011)
Article DOI: 10.1016/j.bmcl.2011.07.039
BindingDB Entry DOI: 10.7270/Q2CR5TR9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50118522
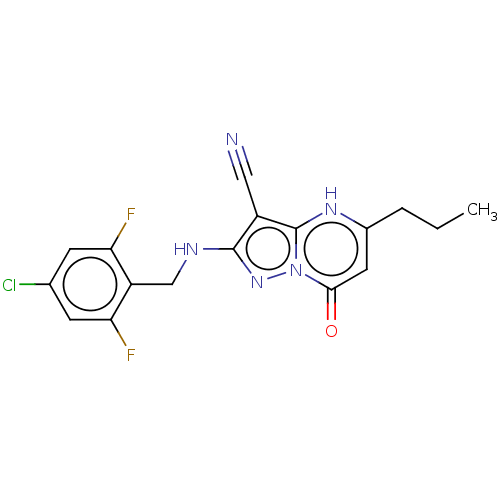
(CHEMBL3617078)Show SMILES CCCc1cc(=O)n2nc(NCc3c(F)cc(Cl)cc3F)c(C#N)c2[nH]1 Show InChI InChI=1S/C17H14ClF2N5O/c1-2-3-10-6-15(26)25-17(23-10)11(7-21)16(24-25)22-8-12-13(19)4-9(18)5-14(12)20/h4-6,23H,2-3,8H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D
Curated by ChEMBL
| Assay Description
Inhibition of BCATc (unknown origin) |
J Med Chem 58: 7140-63 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00313
BindingDB Entry DOI: 10.7270/Q2T43VWK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607290
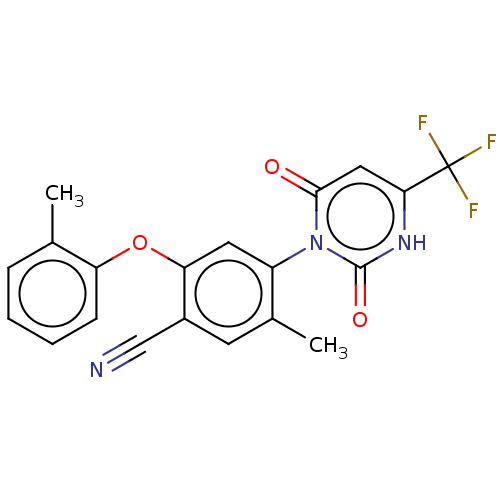
(CHEMBL5220488)Show SMILES Cc1ccccc1Oc1cc(c(C)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.33,5,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;3.33,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.67,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.67,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 287 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607283
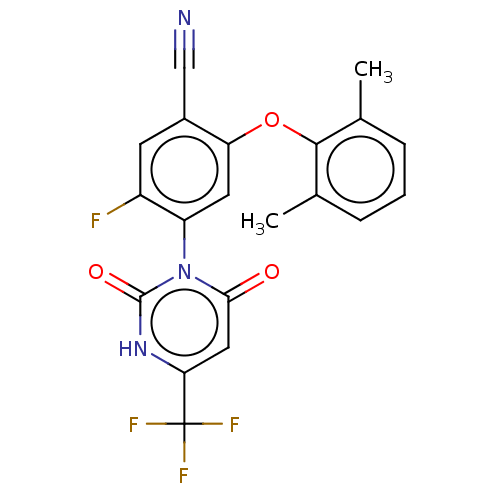
(CHEMBL5219683)Show SMILES Cc1cccc(C)c1Oc1cc(c(F)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;.66,5,;-.67,5.77,;-2,5,;-2,3.47,;-.67,2.69,;-.67,1.15,;.67,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607284
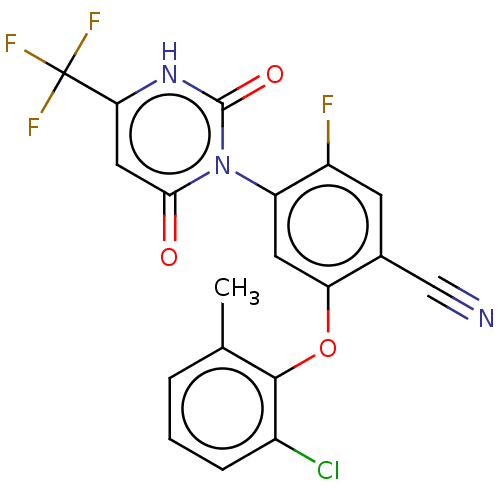
(CHEMBL5218519)Show SMILES Cc1cccc(Cl)c1Oc1cc(c(F)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(-.67,1.15,;-.67,2.69,;-2,3.47,;-2,5,;-.67,5.77,;.66,5,;2,5.77,;.67,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607292
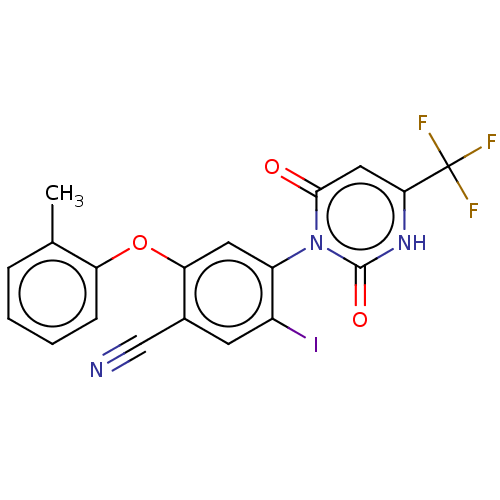
(CHEMBL5219699)Show SMILES Cc1ccccc1Oc1cc(c(I)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.33,5,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;3.33,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.67,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.67,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 413 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607310

(CHEMBL5220057)Show SMILES COc1cc(Cl)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)F |(3.34,-4.24,;2.01,-3.47,;2.01,-1.93,;3.34,-1.15,;3.34,.38,;4.67,1.15,;2,1.15,;2,2.69,;3.34,3.46,;4.68,2.69,;6,3.47,;6,5,;4.67,5.77,;3.34,5,;2,5.77,;.67,.39,;.67,-1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.66,-4.23,;-4.66,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 438 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607289

(CHEMBL5219137)Show SMILES Cc1ccccc1Oc1cc(ccc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 507 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607288

(CHEMBL5220817)Show SMILES Cc1ccccc1Oc1cc(c(F)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.33,5,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;3.33,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.67,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.67,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 554 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607302
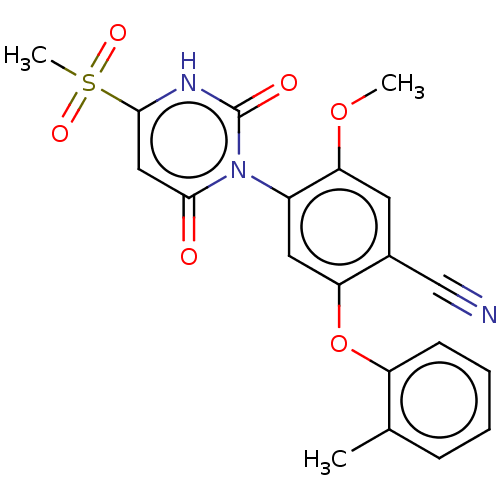
(CHEMBL5219393)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)S(C)(=O)=O |(3.44,-4.24,;2.11,-3.47,;2.11,-1.93,;3.44,-1.15,;3.44,.38,;4.77,1.15,;6.1,1.92,;2.1,1.15,;2.1,2.69,;3.44,3.46,;4.78,2.69,;6.1,3.47,;6.1,5,;4.77,5.77,;3.44,5,;2.1,5.77,;.77,.39,;.77,-1.15,;-.56,-1.92,;-1.9,-1.15,;-1.9,.39,;-3.23,-1.92,;-3.23,-3.46,;-1.9,-4.23,;-.56,-3.46,;.77,-4.23,;-4.56,-4.23,;-4.56,-5.77,;-6.1,-4.23,;-4.96,-2.75,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607296
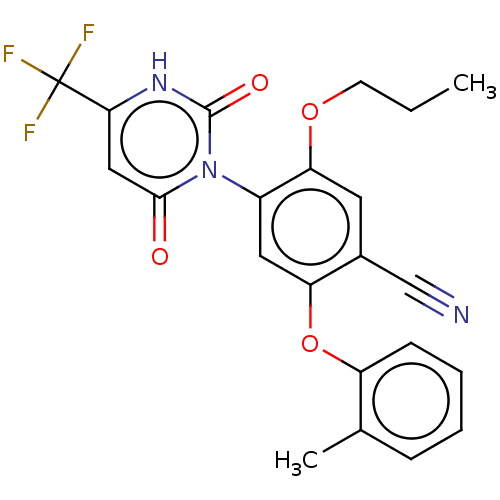
(CHEMBL5219459)Show SMILES CCCOc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)F |(4.68,-6.16,;3.34,-5.39,;3.34,-3.85,;2.01,-3.08,;2.01,-1.54,;3.34,-.76,;3.33,.77,;4.67,1.54,;6,2.31,;2,1.54,;2,3.08,;3.33,3.85,;4.67,3.08,;6,3.85,;6,5.39,;4.66,6.16,;3.33,5.39,;2,6.16,;.67,.77,;.67,-.77,;-.66,-1.54,;-2,-.77,;-2,.77,;-3.33,-1.54,;-3.33,-3.08,;-2,-3.85,;-.66,-3.08,;.67,-3.85,;-4.67,-3.85,;-4.67,-5.39,;-6,-3.08,;-6,-4.62,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 723 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607305
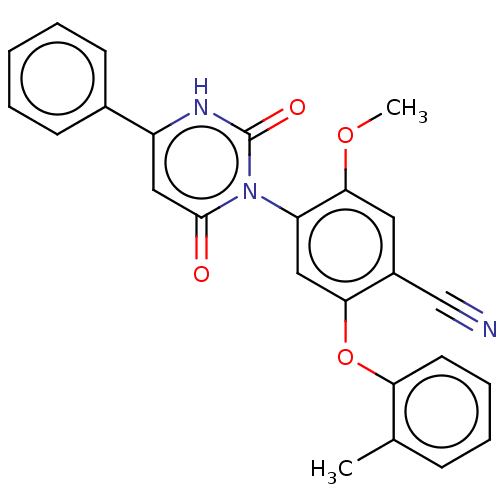
(CHEMBL5220789)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)-c1ccccc1 |(4.01,-3.85,;2.67,-3.08,;2.67,-1.54,;4,-.77,;4,.77,;5.33,1.54,;6.67,2.31,;2.67,1.54,;2.67,3.08,;4,3.85,;5.34,3.07,;6.67,3.85,;6.66,5.38,;5.33,6.15,;4,5.39,;2.67,6.16,;1.34,.77,;1.34,-.77,;0,-1.54,;-1.33,-.77,;-1.33,.77,;-2.67,-1.54,;-2.67,-3.08,;-1.33,-3.85,;0,-3.08,;1.34,-3.85,;-4,-3.85,;-4,-5.39,;-5.33,-6.16,;-6.66,-5.39,;-6.67,-3.85,;-5.34,-3.08,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 725 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607299
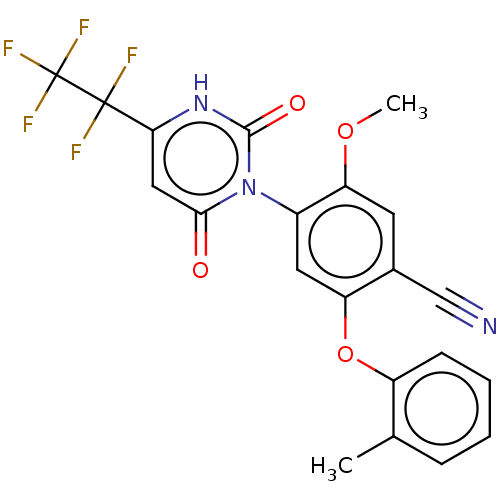
(CHEMBL5219673)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)C(F)(F)F |(3.42,-3.47,;2.08,-2.7,;2.08,-1.16,;3.41,-.38,;3.41,1.15,;4.74,1.92,;6.08,2.69,;2.08,1.92,;2.08,3.46,;3.41,4.23,;4.75,3.46,;6.08,4.24,;6.08,5.77,;4.74,6.54,;3.41,5.77,;2.08,6.54,;.75,1.16,;.75,-.38,;-.59,-1.15,;-1.92,-.38,;-1.92,1.16,;-3.26,-1.15,;-3.26,-2.69,;-1.92,-3.46,;-.59,-2.69,;.75,-3.46,;-4.59,-3.46,;-6.08,-3.86,;-5.36,-2.13,;-4.59,-5,;-5.92,-5.77,;-3.26,-5.77,;-4.59,-6.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 792 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172933

(5-chloro-N'-(2-(trifluoromethyl)phenylsulfonyl)-1H...)Show SMILES FC(F)(F)c1ccccc1S(=O)(=O)NNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C16H11ClF3N3O3S/c17-10-5-6-12-9(7-10)8-13(21-12)15(24)22-23-27(25,26)14-4-2-1-3-11(14)16(18,19)20/h1-8,21,23H,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607281

(CHEMBL5218966)Show SMILES Fc1cccc(F)c1Oc1cc(c(F)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;.66,5,;-.67,5.77,;-2,5,;-2,3.47,;-.67,2.69,;-.67,1.15,;.67,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607318
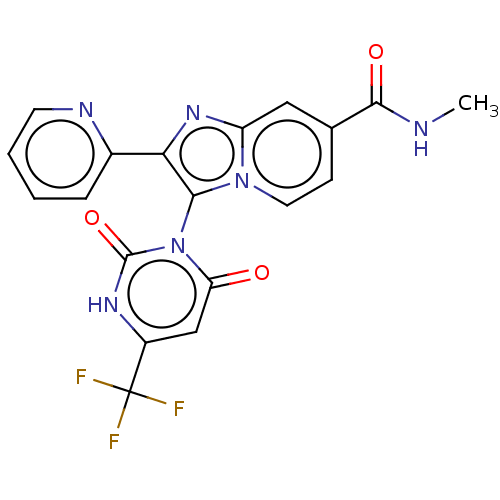
(CHEMBL5220587)Show SMILES CNC(=O)c1ccn2c(c(nc2c1)-c1ccccn1)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(-6.83,4.34,;-5.49,3.57,;-4.16,4.34,;-4.16,5.88,;-2.83,3.57,;-2.83,2.03,;-1.49,1.26,;-.16,2.03,;1.31,1.56,;2.21,2.8,;1.31,4.05,;-.16,3.57,;-1.49,4.34,;3.75,2.8,;4.52,4.14,;6.06,4.13,;6.83,2.8,;6.06,1.47,;4.52,1.46,;1.7,.07,;.62,-1.02,;-.87,-.62,;1.01,-2.51,;2.5,-2.91,;3.59,-1.82,;3.19,-.33,;4.28,.76,;2.9,-4.39,;4.39,-4.79,;1.41,-4.79,;2.5,-5.88,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607317
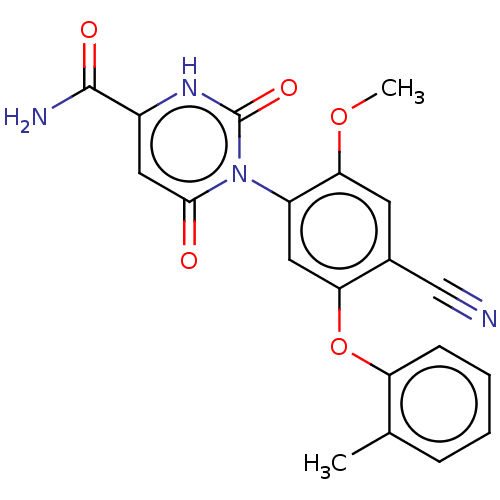
(CHEMBL5220979)Show SMILES COc1cc(C#N)c(Oc2ccccc2C)cc1-n1c(=O)cc([nH]c1=O)C(N)=O |(3.34,-4.22,;2.01,-3.46,;2.01,-1.92,;3.33,-1.15,;3.33,.39,;4.67,1.16,;6,1.93,;2,1.15,;2,2.69,;.67,3.46,;-.66,2.69,;-1.99,3.46,;-1.99,5,;-.67,5.77,;.67,5.01,;2,5.78,;.67,.39,;.67,-1.16,;-.66,-1.93,;-2,-1.16,;-2,.38,;-3.33,-1.93,;-3.33,-3.47,;-2,-4.24,;-.66,-3.47,;.67,-4.24,;-4.67,-4.24,;-6,-3.47,;-4.67,-5.78,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607295
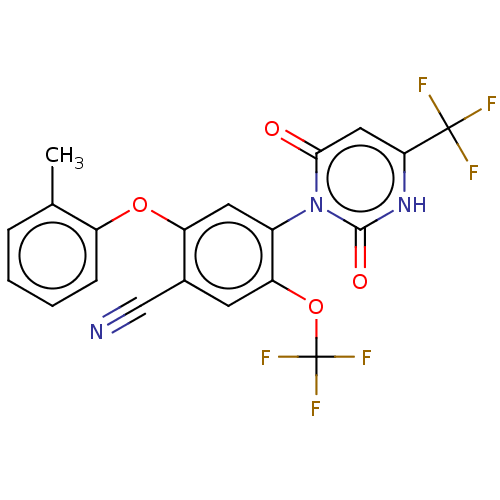
(CHEMBL5219169)Show SMILES Cc1ccccc1Oc1cc(c(OC(F)(F)F)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.33,5,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;3.33,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.92,;2.01,-3.46,;3.34,-4.23,;3.34,-5.77,;4.67,-3.46,;4.67,-5,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 925 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607285
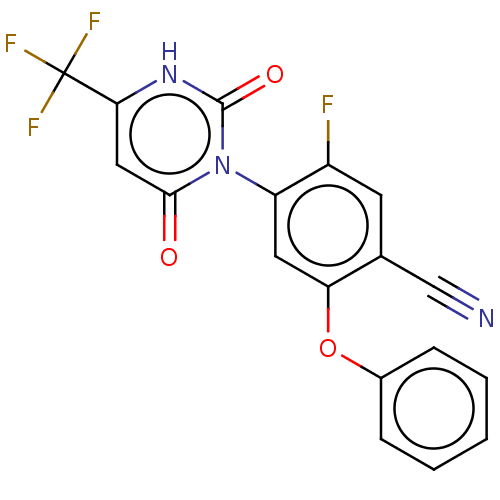
(CHEMBL5219665)Show SMILES Fc1cc(C#N)c(Oc2ccccc2)cc1-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2.01,-3.46,;2.01,-1.92,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;2,1.15,;2,2.69,;3.33,3.47,;3.33,5.01,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;.67,.39,;.67,-1.15,;-.67,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.67,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172943
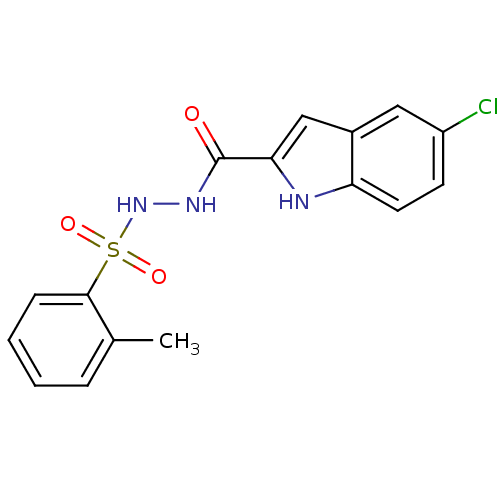
(5-chloro-N'-(o-tolylsulfonyl)-1H-indole-2-carbohyd...)Show SMILES Cc1ccccc1S(=O)(=O)NNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C16H14ClN3O3S/c1-10-4-2-3-5-15(10)24(22,23)20-19-16(21)14-9-11-8-12(17)6-7-13(11)18-14/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172934
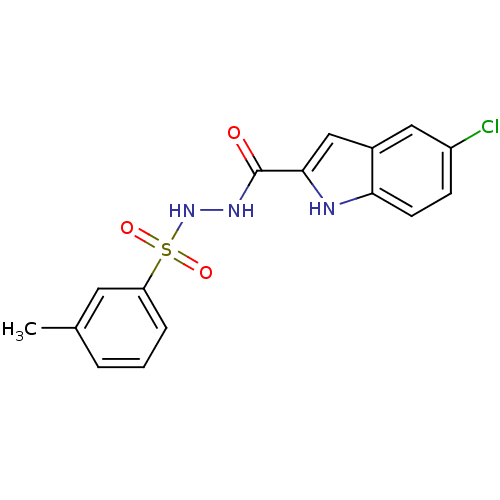
(5-chloro-N'-(m-tolylsulfonyl)-1H-indole-2-carbohyd...)Show SMILES Cc1cccc(c1)S(=O)(=O)NNC(=O)c1cc2cc(Cl)ccc2[nH]1 Show InChI InChI=1S/C16H14ClN3O3S/c1-10-3-2-4-13(7-10)24(22,23)20-19-16(21)15-9-11-8-12(17)5-6-14(11)18-15/h2-9,18,20H,1H3,(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50173939

(CHEMBL3809237)Show SMILES CNC(=O)c1ccc2n([C@@H]3CCC[C@@H](C3)NC(=O)c3ccc(Br)s3)c(nc2c1)-c1ccccc1SC |r| Show InChI InChI=1S/C27H27BrN4O2S2/c1-29-26(33)16-10-11-21-20(14-16)31-25(19-8-3-4-9-22(19)35-2)32(21)18-7-5-6-17(15-18)30-27(34)23-12-13-24(28)36-23/h3-4,8-14,17-18H,5-7,15H2,1-2H3,(H,29,33)(H,30,34)/t17-,18+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of BCATc (unknown origin) |
ACS Med Chem Lett 7: 379-84 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00389
BindingDB Entry DOI: 10.7270/Q2W097V6 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607293
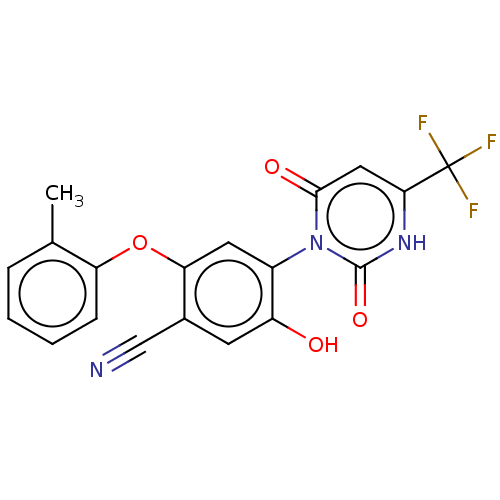
(CHEMBL5219930)Show SMILES Cc1ccccc1Oc1cc(c(O)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.33,5,;4.66,5.77,;6,5,;6,3.47,;4.67,2.69,;3.33,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.33,.38,;4.67,1.15,;6,1.92,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.67,-4.23,;-4.67,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607282
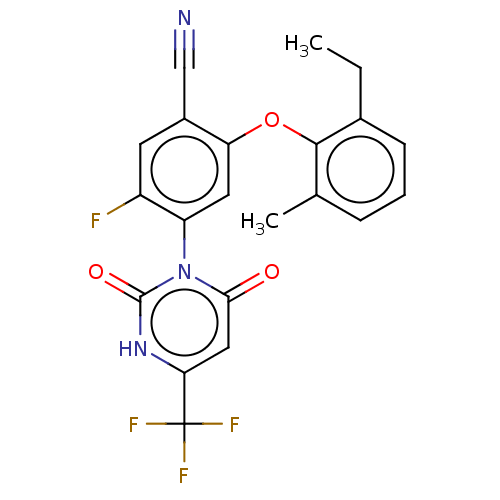
(CHEMBL5218682)Show SMILES CCc1cccc(C)c1Oc1cc(c(F)cc1C#N)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,6.54,;2,5,;.66,4.23,;-.67,5,;-2,4.23,;-2,2.7,;-.67,1.92,;-.67,.38,;.67,2.69,;2,1.92,;2,.38,;.67,-.38,;.67,-1.92,;2.01,-2.7,;2.01,-4.24,;3.34,-1.92,;3.33,-.39,;4.67,.38,;6,1.15,;-.67,-2.69,;-2,-1.92,;-2,-.38,;-3.33,-2.69,;-3.33,-4.23,;-2,-5,;-.67,-4.23,;.67,-5,;-4.67,-5,;-4.67,-6.54,;-6,-4.23,;-6,-5.77,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607308
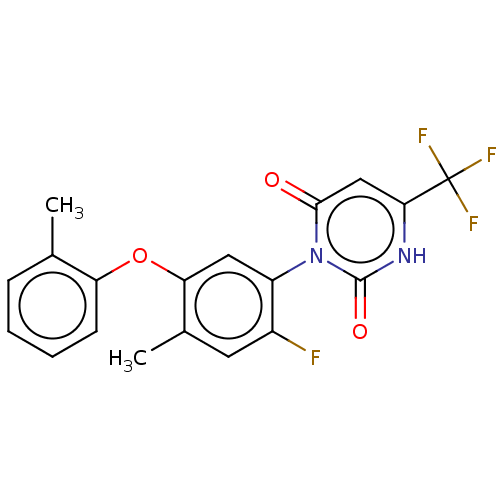
(CHEMBL5219636)Show SMILES Cc1ccccc1Oc1cc(c(F)cc1C)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(2,5.77,;3.34,5,;4.67,5.77,;6,5,;6,3.47,;4.68,2.69,;3.34,3.46,;2,2.69,;2,1.15,;.67,.39,;.67,-1.15,;2.01,-1.93,;2.01,-3.47,;3.34,-1.15,;3.34,.38,;4.67,1.15,;-.66,-1.92,;-2,-1.15,;-2,.39,;-3.33,-1.92,;-3.33,-3.46,;-2,-4.23,;-.66,-3.46,;.67,-4.23,;-4.66,-4.23,;-4.66,-5.77,;-6,-3.46,;-6,-5,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172945
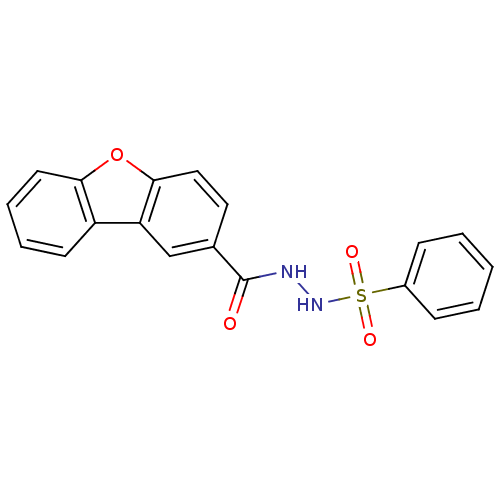
(CHEMBL205902 | N'-(phenylsulfonyl)dibenzo[b,d]fura...)Show SMILES O=C(NNS(=O)(=O)c1ccccc1)c1ccc2oc3ccccc3c2c1 Show InChI InChI=1S/C19H14N2O4S/c22-19(20-21-26(23,24)14-6-2-1-3-7-14)13-10-11-18-16(12-13)15-8-4-5-9-17(15)25-18/h1-12,21H,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172944
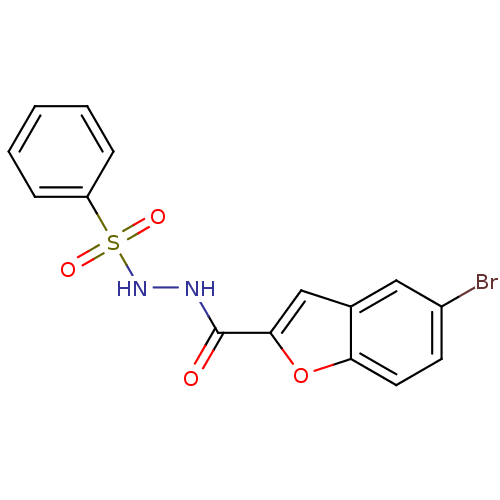
(5-bromo-N'-(phenylsulfonyl)benzofuran-2-carbohydra...)Show InChI InChI=1S/C15H11BrN2O4S/c16-11-6-7-13-10(8-11)9-14(22-13)15(19)17-18-23(20,21)12-4-2-1-3-5-12/h1-9,18H,(H,17,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172940
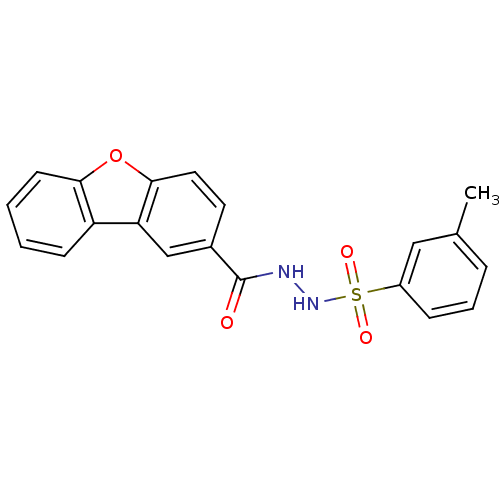
(CHEMBL437702 | N'-[(3-methylphenyl)sulfonyl]dibenz...)Show SMILES Cc1cccc(c1)S(=O)(=O)NNC(=O)c1ccc2oc3ccccc3c2c1 Show InChI InChI=1S/C20H16N2O4S/c1-13-5-4-6-15(11-13)27(24,25)22-21-20(23)14-9-10-19-17(12-14)16-7-2-3-8-18(16)26-19/h2-12,22H,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50172931

(CHEMBL205475 | N'-[(2-methylphenyl)sulfonyl]dibenz...)Show SMILES Cc1ccccc1S(=O)(=O)NNC(=O)c1ccc2oc3ccccc3c2c1 Show InChI InChI=1S/C20H16N2O4S/c1-13-6-2-5-9-19(13)27(24,25)22-21-20(23)14-10-11-18-16(12-14)15-7-3-4-8-17(15)26-18/h2-12,22H,1H3,(H,21,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human BCATc |
Bioorg Med Chem Lett 16: 2337-40 (2006)
Article DOI: 10.1016/j.bmcl.2005.07.058
BindingDB Entry DOI: 10.7270/Q2F47NQZ |
More data for this
Ligand-Target Pair | |
Branched-chain-amino-acid aminotransferase, cytosolic
(Homo sapiens (Human)) | BDBM50607287

(CHEMBL5219727)Show SMILES COc1cc(OC)c(cc1Cl)-n1c(=O)cc([nH]c1=O)C(F)(F)F |(5.33,4.23,;5.33,2.69,;4,1.92,;4,.39,;2.67,-.39,;2.67,-1.93,;4.01,-2.7,;1.33,.39,;1.34,1.93,;2.67,2.69,;2.67,4.23,;0,-.38,;-1.33,.39,;-1.33,1.93,;-2.67,-.38,;-2.67,-1.92,;-1.33,-2.69,;0,-1.92,;1.33,-2.69,;-4,-2.69,;-4,-4.23,;-5.33,-1.92,;-5.33,-3.46,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00441
BindingDB Entry DOI: 10.7270/Q2SN0F31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data