 Found 39 hits of ic50 for UniProtKB: P00642
Found 39 hits of ic50 for UniProtKB: P00642 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250738

(Acetic acid (1R,5aR,8R,9R,10aS,11R,12R,12aR)-1-((R...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@H]2OC(C)(C)O[C@@H]2C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C35H54O5/c1-19(2)20(3)12-13-21(4)24-15-16-25-23-14-17-27-32(6,7)30-26(39-33(8,9)40-30)18-34(27,10)28(23)29(37)31(35(24,25)11)38-22(5)36/h16,19,21,24,26-27,29-31,37H,3,12-15,17-18H2,1-2,4-11H3/t21-,24-,26-,27+,29-,30+,31+,34+,35-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250756
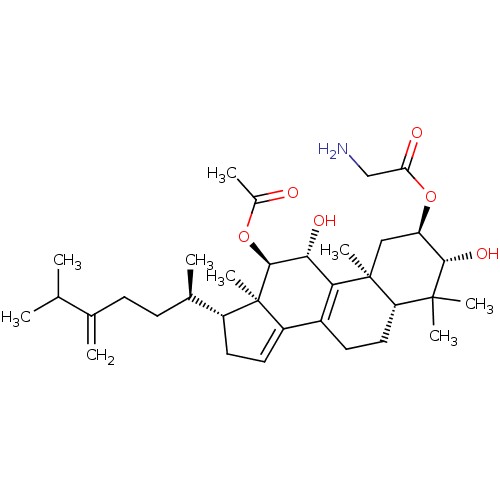
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CN)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C34H53NO6/c1-18(2)19(3)10-11-20(4)23-13-14-24-22-12-15-26-32(6,7)30(39)25(41-27(37)17-35)16-33(26,8)28(22)29(38)31(34(23,24)9)40-21(5)36/h14,18,20,23,25-26,29-31,38-39H,3,10-13,15-17,35H2,1-2,4-9H3/t20-,23-,25-,26+,29-,30+,31+,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250755
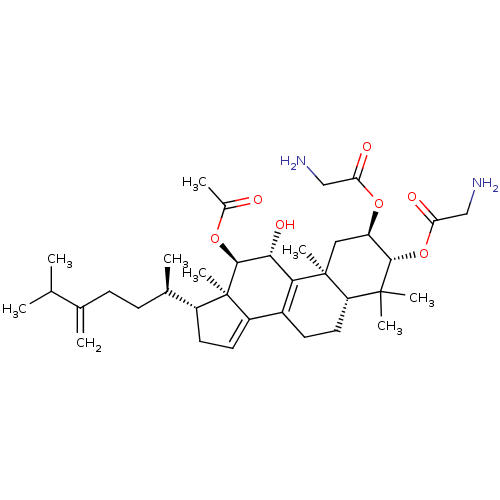
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CN)[C@H](OC(=O)CN)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H56N2O7/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(45-29(41)18-38)26(44-28(40)17-37)16-35(27,8)30(23)31(42)33(36(24,25)9)43-22(5)39/h14,19,21,24,26-27,31-33,42H,3,10-13,15-18,37-38H2,1-2,4-9H3/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250754

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CNC(=O)OCC2c4ccccc4-c4ccccc24)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C49H63NO8/c1-27(2)28(3)18-19-29(4)37-21-22-38-35-20-23-40-47(6,7)44(54)39(24-48(40,8)42(35)43(53)45(49(37,38)9)57-30(5)51)58-41(52)25-50-46(55)56-26-36-33-16-12-10-14-31(33)32-15-11-13-17-34(32)36/h10-17,22,27,29,36-37,39-40,43-45,53-54H,3,18-21,23-26H2,1-2,4-9H3,(H,50,55)/t29-,37-,39-,40+,43-,44+,45+,48+,49-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250712
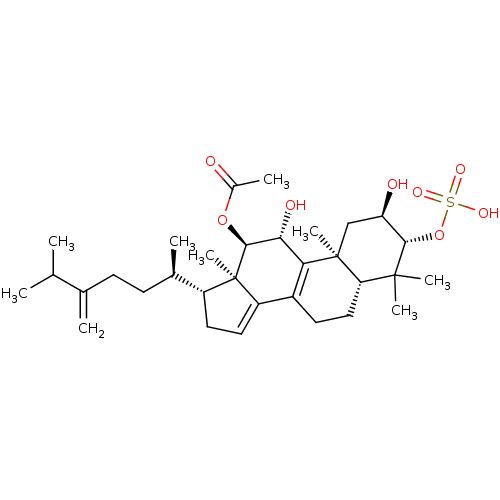
(CHEMBL446297 | Integracide A)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H50O8S/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(40-41(36,37)38)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)39-20(5)33/h14,17,19,22,24-25,27-29,34-35H,3,10-13,15-16H2,1-2,4-9H3,(H,36,37,38)/t19-,22-,24-,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250713
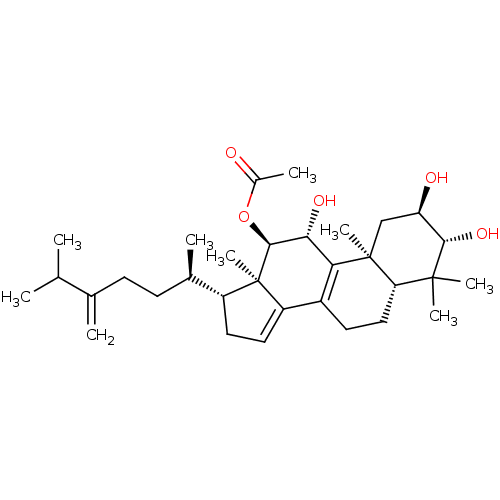
(CHEMBL464128 | Integracide B)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H50O5/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(36)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)37-20(5)33/h14,17,19,22,24-25,27-29,34-36H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250714

((2R,3R,5R,10S,11R,12R,13R,14S,17R)-2,11-dihydroxy-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC[C@H]2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:13| Show InChI InChI=1S/C32H52O8S/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(40-41(36,37)38)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)39-20(5)33/h17,19,22-25,27-29,34-35H,3,10-16H2,1-2,4-9H3,(H,36,37,38)/t19-,22-,23+,24-,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250715
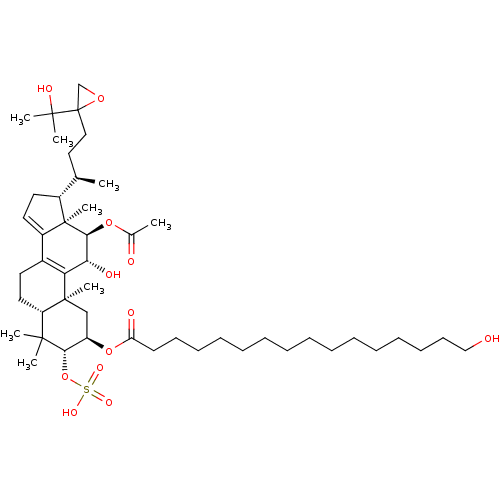
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES C[C@H](CCC1(CO1)C(C)(C)O)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCCCCCCCCCCCCCCO)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:16,t:14| Show InChI InChI=1S/C48H80O12S/c1-32(27-28-48(31-57-48)45(5,6)53)35-24-25-36-34-23-26-38-44(3,4)42(60-61(54,55)56)37(30-46(38,7)40(34)41(52)43(47(35,36)8)58-33(2)50)59-39(51)22-20-18-16-14-12-10-9-11-13-15-17-19-21-29-49/h25,32,35,37-38,41-43,49,52-53H,9-24,26-31H2,1-8H3,(H,54,55,56)/t32-,35-,37-,38+,41-,42+,43+,46+,47-,48?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250716

((2R,3R,5R,10S,11R,12R,13R,14S,17R)-12-acetoxy-11-h...)Show SMILES C[C@H](CCC1(CO1)C(C)(C)O)[C@H]1CC[C@H]2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCCCCCCCCCCCCCCO)[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:16| Show InChI InChI=1S/C48H82O12S/c1-32(27-28-48(31-57-48)45(5,6)53)35-24-25-36-34-23-26-38-44(3,4)42(60-61(54,55)56)37(30-46(38,7)40(34)41(52)43(47(35,36)8)58-33(2)50)59-39(51)22-20-18-16-14-12-10-9-11-13-15-17-19-21-29-49/h32,35-38,41-43,49,52-53H,9-31H2,1-8H3,(H,54,55,56)/t32-,35-,36+,37-,38+,41-,42+,43+,46+,47-,48?/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250717
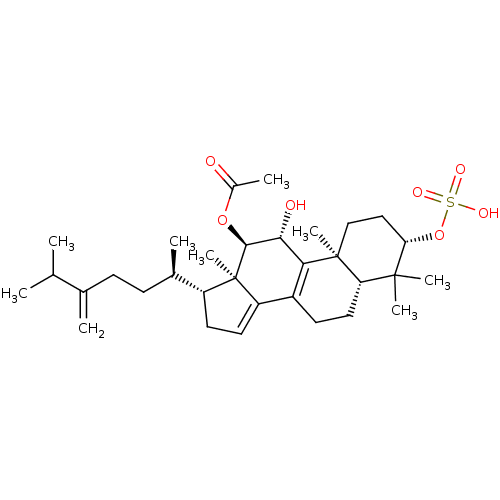
((3S,5R,10S,11R,12R,13R,17R)-11-hydroxy-4,4,10,13-t...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)CC[C@H](OS(O)(=O)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H50O7S/c1-18(2)19(3)10-11-20(4)23-13-14-24-22-12-15-25-30(6,7)26(39-40(35,36)37)16-17-31(25,8)27(22)28(34)29(32(23,24)9)38-21(5)33/h14,18,20,23,25-26,28-29,34H,3,10-13,15-17H2,1-2,4-9H3,(H,35,36,37)/t20-,23-,25+,26+,28-,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250718

(2-alpha-(3'-hydroxy-3'-methylglutaroyl)-24,25-dihy...)Show SMILES C[C@H](CCC(O)C(C)(C)O)[C@H]1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CC(C)(O)CC(O)=O)C(=O)C(C)(C)[C@@H]1CC3 |r,c:15| Show InChI InChI=1S/C36H58O8/c1-21(10-13-27(37)32(4,5)42)22-14-16-36(9)24-11-12-26-31(2,3)30(41)25(44-29(40)20-33(6,43)19-28(38)39)18-34(26,7)23(24)15-17-35(22,36)8/h21-22,25-27,37,42-43H,10-20H2,1-9H3,(H,38,39)/t21-,22-,25-,26+,27?,33?,34-,35-,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250719
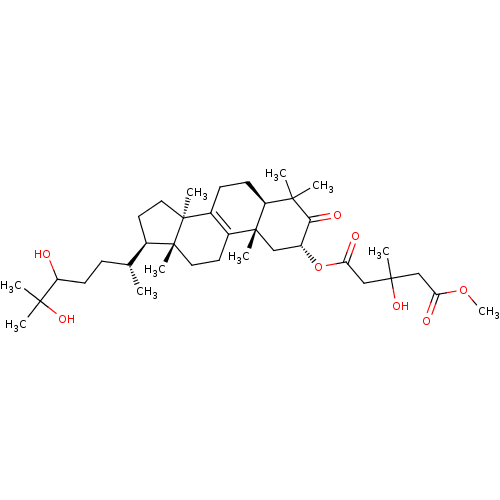
((3R)-1-((2R,5R,10S,13R,14R,17R)-17-((2R)-5,6-dihyd...)Show SMILES COC(=O)CC(C)(O)CC(=O)O[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2CC[C@]2(C)[C@H](CC[C@@]32C)[C@H](C)CCC(O)C(C)(C)O)C(C)(C)C1=O |r,c:19| Show InChI InChI=1S/C37H60O8/c1-22(11-14-28(38)33(4,5)42)23-15-17-37(9)25-12-13-27-32(2,3)31(41)26(19-35(27,7)24(25)16-18-36(23,37)8)45-30(40)21-34(6,43)20-29(39)44-10/h22-23,26-28,38,42-43H,11-21H2,1-10H3/t22-,23-,26-,27+,28?,34?,35-,36-,37+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250727
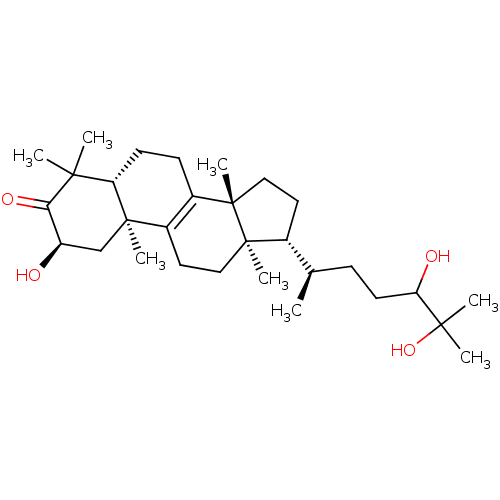
((2R,5R,10S,13R,14R,17R)-17-((2R)-5,6-dihydroxy-6-m...)Show SMILES C[C@H](CCC(O)C(C)(C)O)[C@H]1CC[C@@]2(C)C3=C(CC[C@]12C)[C@@]1(C)C[C@@H](O)C(=O)C(C)(C)[C@@H]1CC3 |r,c:15| Show InChI InChI=1S/C30H50O4/c1-18(9-12-24(32)27(4,5)34)19-13-15-30(8)21-10-11-23-26(2,3)25(33)22(31)17-28(23,6)20(21)14-16-29(19,30)7/h18-19,22-24,31-32,34H,9-17H2,1-8H3/t18-,19-,22-,23+,24?,28-,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250728
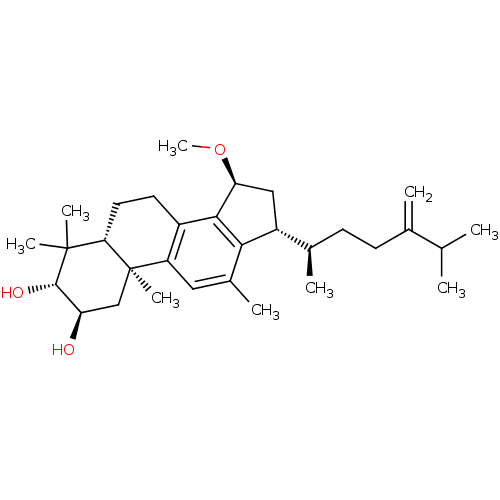
((2R,3R,5R,10S,15S,17R)-15-methoxy-4,4,10,12-tetram...)Show SMILES CO[C@H]1C[C@H]([C@H](C)CCC(=C)C(C)C)c2c1c1CC[C@H]3C(C)(C)[C@@H](O)[C@H](O)C[C@]3(C)c1cc2C |r| Show InChI InChI=1S/C31H48O3/c1-17(2)18(3)10-11-19(4)22-15-25(34-9)28-21-12-13-26-30(6,7)29(33)24(32)16-31(26,8)23(21)14-20(5)27(22)28/h14,17,19,22,24-26,29,32-33H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25+,26+,29+,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250729
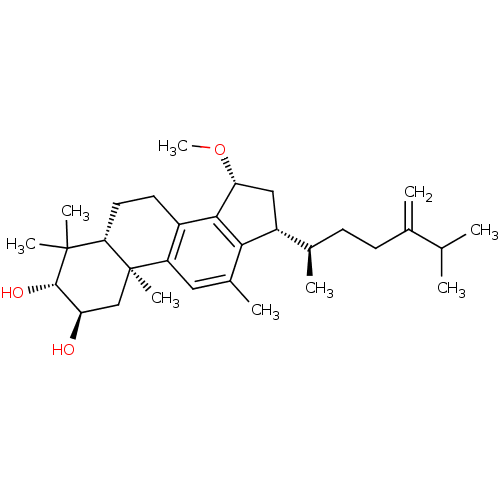
((2R,3R,5R,10S,15R,17R)-15-methoxy-4,4,10,12-tetram...)Show SMILES CO[C@@H]1C[C@H]([C@H](C)CCC(=C)C(C)C)c2c1c1CC[C@H]3C(C)(C)[C@@H](O)[C@H](O)C[C@]3(C)c1cc2C |r| Show InChI InChI=1S/C31H48O3/c1-17(2)18(3)10-11-19(4)22-15-25(34-9)28-21-12-13-26-30(6,7)29(33)24(32)16-31(26,8)23(21)14-20(5)27(22)28/h14,17,19,22,24-26,29,32-33H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25-,26+,29+,31-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250730
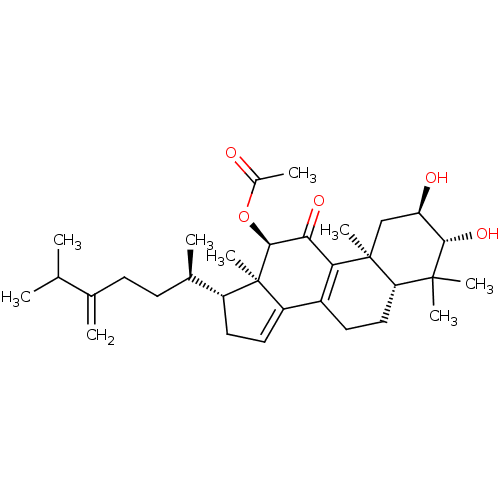
((2R,3R,5R,10S,12R,13R,17R)-2,3-dihydroxy-4,4,10,13...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C(C(=O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H48O5/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28(36)24(34)16-31(25,8)26(21)27(35)29(32(22,23)9)37-20(5)33/h14,17,19,22,24-25,28-29,34,36H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24-,25+,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250735
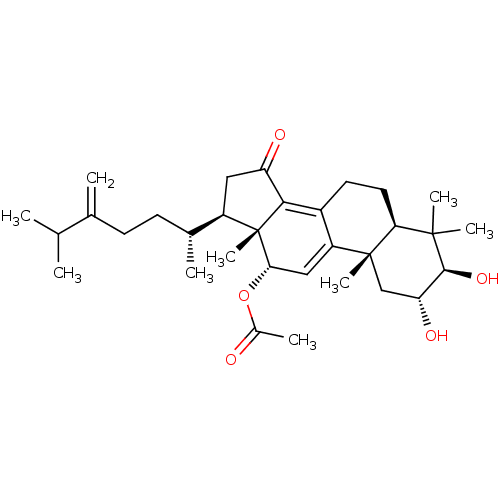
((2R,3R,5R,10S,12S,13R,17R)-2,3-dihydroxy-4,4,10,13...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC(=O)C2=C3CC[C@H]4C(C)(C)[C@@H](O)[C@H](O)C[C@]4(C)C3=C[C@H](OC(C)=O)[C@]12C |r,c:13,30| Show InChI InChI=1S/C32H48O5/c1-17(2)18(3)10-11-19(4)22-14-24(34)28-21-12-13-26-30(6,7)29(36)25(35)16-31(26,8)23(21)15-27(32(22,28)9)37-20(5)33/h15,17,19,22,25-27,29,35-36H,3,10-14,16H2,1-2,4-9H3/t19-,22-,25-,26+,27+,29+,31-,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250736
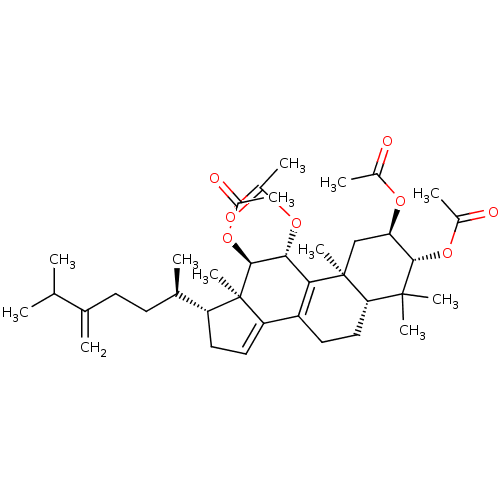
((2R,3R,5R,10S,11R,12R,13R,17R)-4,4,10,13-tetrameth...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](OC(C)=O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(C)=O)[C@H](OC(C)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C38H56O8/c1-20(2)21(3)13-14-22(4)28-16-17-29-27-15-18-31-36(9,10)34(45-25(7)41)30(43-23(5)39)19-37(31,11)32(27)33(44-24(6)40)35(38(28,29)12)46-26(8)42/h17,20,22,28,30-31,33-35H,3,13-16,18-19H2,1-2,4-12H3/t22-,28-,30-,31+,33-,34+,35+,37+,38-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250737
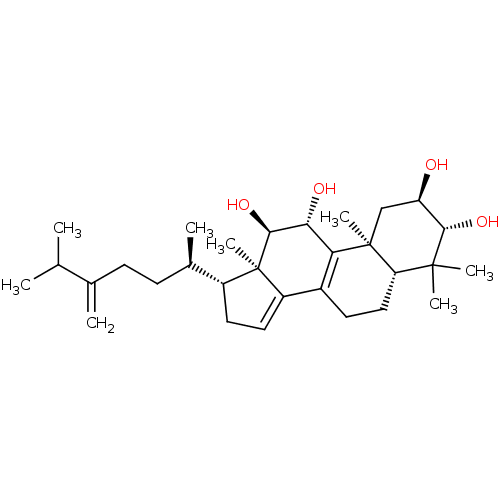
((2R,3R,5R,10S,11R,12R,13R,17R)-4,4,10,13-tetrameth...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C30H48O4/c1-16(2)17(3)9-10-18(4)20-12-13-21-19-11-14-23-28(5,6)26(33)22(31)15-29(23,7)24(19)25(32)27(34)30(20,21)8/h13,16,18,20,22-23,25-27,31-34H,3,9-12,14-15H2,1-2,4-8H3/t18-,20-,22-,23+,25-,26+,27+,29+,30-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250757
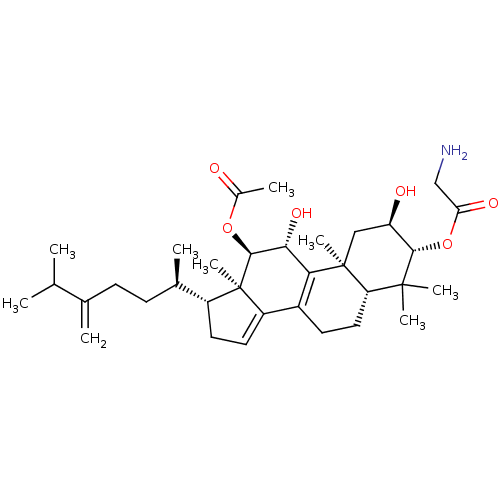
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OC(=O)CN)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C34H53NO6/c1-18(2)19(3)10-11-20(4)23-13-14-24-22-12-15-26-32(6,7)30(41-27(38)17-35)25(37)16-33(26,8)28(22)29(39)31(34(23,24)9)40-21(5)36/h14,18,20,23,25-26,29-31,37,39H,3,10-13,15-17,35H2,1-2,4-9H3/t20-,23-,25-,26+,29-,30+,31+,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250739

(Acetic acid (5R,10S,11R,12R,13R,17R)-17-((R)-1,5-d...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H]2O[C@@H]2C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C32H48O4/c1-17(2)18(3)10-11-19(4)22-13-14-23-21-12-15-25-30(6,7)28-24(36-28)16-31(25,8)26(21)27(34)29(32(22,23)9)35-20(5)33/h14,17,19,22,24-25,27-29,34H,3,10-13,15-16H2,1-2,4-9H3/t19-,22-,24+,25+,27-,28+,29+,31+,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250740

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)c2ccccc2)[C@H](OC(=O)c2ccccc2)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C46H58O7/c1-27(2)28(3)20-21-29(4)34-23-24-35-33-22-25-37-44(6,7)40(53-43(50)32-18-14-11-15-19-32)36(52-42(49)31-16-12-10-13-17-31)26-45(37,8)38(33)39(48)41(46(34,35)9)51-30(5)47/h10-19,24,27,29,34,36-37,39-41,48H,3,20-23,25-26H2,1-2,4-9H3/t29-,34-,36-,37+,39-,40+,41+,45+,46-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250741
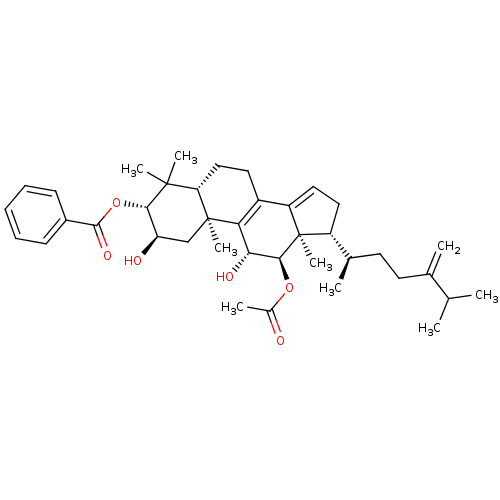
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OC(=O)c2ccccc2)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C39H54O6/c1-22(2)23(3)15-16-24(4)28-18-19-29-27-17-20-31-37(6,7)34(45-36(43)26-13-11-10-12-14-26)30(41)21-38(31,8)32(27)33(42)35(39(28,29)9)44-25(5)40/h10-14,19,22,24,28,30-31,33-35,41-42H,3,15-18,20-21H2,1-2,4-9H3/t24-,28-,30-,31+,33-,34+,35+,38+,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250742
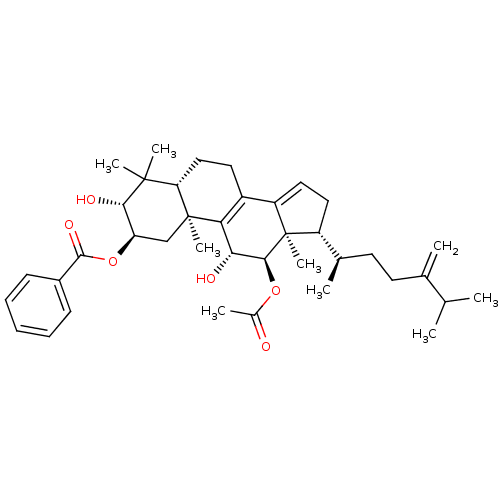
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)c2ccccc2)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C39H54O6/c1-22(2)23(3)15-16-24(4)28-18-19-29-27-17-20-31-37(6,7)34(42)30(45-36(43)26-13-11-10-12-14-26)21-38(31,8)32(27)33(41)35(39(28,29)9)44-25(5)40/h10-14,19,22,24,28,30-31,33-35,41-42H,3,15-18,20-21H2,1-2,4-9H3/t24-,28-,30-,31+,33-,34+,35+,38+,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250743

((2R,3R,5R,10S,11R,12R,13R,17R)-11-hydroxy-2,3-bis(...)Show SMILES COCO[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C(C)(C)[C@H]1OCOC |r,c:11,t:25| Show InChI InChI=1S/C36H58O7/c1-21(2)22(3)12-13-23(4)26-15-16-27-25-14-17-29-34(6,7)32(42-20-40-11)28(41-19-39-10)18-35(29,8)30(25)31(38)33(36(26,27)9)43-24(5)37/h16,21,23,26,28-29,31-33,38H,3,12-15,17-20H2,1-2,4-11H3/t23-,26-,28-,29+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250744
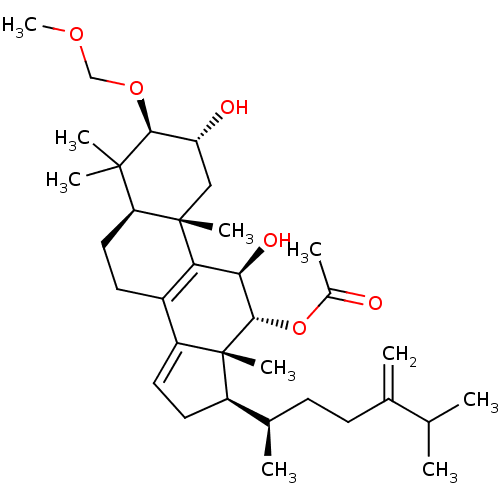
((2R,3R,5R,10S,11R,12R,13R,17R)-2,11-dihydroxy-3-(m...)Show SMILES COCO[C@H]1[C@H](O)C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C1(C)C |r,c:13,t:27| Show InChI InChI=1S/C34H54O6/c1-19(2)20(3)11-12-21(4)24-14-15-25-23-13-16-27-32(6,7)30(39-18-38-10)26(36)17-33(27,8)28(23)29(37)31(34(24,25)9)40-22(5)35/h15,19,21,24,26-27,29-31,36-37H,3,11-14,16-18H2,1-2,4-10H3/t21-,24-,26-,27+,29-,30+,31+,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250745
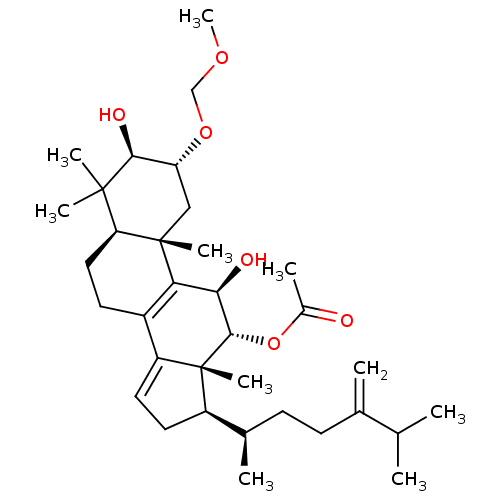
((2R,3R,5R,10S,11R,12R,13R,17R)-3,11-dihydroxy-2-(m...)Show SMILES COCO[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C(C)(C)[C@H]1O |r,c:11,t:25| Show InChI InChI=1S/C34H54O6/c1-19(2)20(3)11-12-21(4)24-14-15-25-23-13-16-27-32(6,7)30(37)26(39-18-38-10)17-33(27,8)28(23)29(36)31(34(24,25)9)40-22(5)35/h15,19,21,24,26-27,29-31,36-37H,3,11-14,16-18H2,1-2,4-10H3/t21-,24-,26-,27+,29-,30+,31+,33+,34-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250746

(4-((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](O)[C@H](OC(=O)CCC(O)=O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H54O8/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(44-29(41)17-16-28(39)40)26(38)18-35(27,8)30(23)31(42)33(36(24,25)9)43-22(5)37/h14,19,21,24,26-27,31-33,38,42H,3,10-13,15-18H2,1-2,4-9H3,(H,39,40)/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250747

(4-((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCC(O)=O)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H54O8/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(42)26(44-29(40)17-16-28(38)39)18-35(27,8)30(23)31(41)33(36(24,25)9)43-22(5)37/h14,19,21,24,26-27,31-33,41-42H,3,10-13,15-18H2,1-2,4-9H3,(H,38,39)/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250748

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-2,11-dih...)Show SMILES COC(=O)CCC(=O)O[C@H]1[C@H](O)C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C1(C)C |r,c:18,t:32| Show InChI InChI=1S/C37H56O8/c1-20(2)21(3)11-12-22(4)25-14-15-26-24-13-16-28-35(6,7)33(45-30(41)18-17-29(40)43-10)27(39)19-36(28,8)31(24)32(42)34(37(25,26)9)44-23(5)38/h15,20,22,25,27-28,32-34,39,42H,3,11-14,16-19H2,1-2,4-10H3/t22-,25-,27-,28+,32-,33+,34+,36+,37-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250749

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES COC(=O)CCC(=O)O[C@@H]1C[C@@]2(C)[C@@H](CCC3=C2[C@@H](O)[C@H](OC(C)=O)[C@]2(C)[C@H](CC=C32)[C@H](C)CCC(=C)C(C)C)C(C)(C)[C@H]1O |r,c:16,t:30| Show InChI InChI=1S/C37H56O8/c1-20(2)21(3)11-12-22(4)25-14-15-26-24-13-16-28-35(6,7)33(42)27(45-30(40)18-17-29(39)43-10)19-36(28,8)31(24)32(41)34(37(25,26)9)44-23(5)38/h15,20,22,25,27-28,32-34,41-42H,3,11-14,16-19H2,1-2,4-10H3/t22-,25-,27-,28+,32-,33+,34+,36+,37-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250750
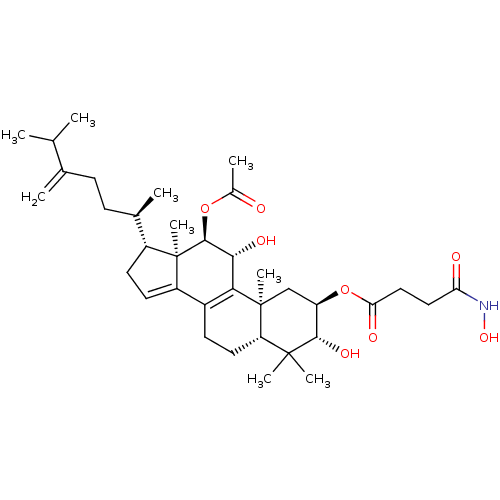
((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CCC(=O)NO)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C36H55NO8/c1-19(2)20(3)10-11-21(4)24-13-14-25-23-12-15-27-34(6,7)32(42)26(45-29(40)17-16-28(39)37-43)18-35(27,8)30(23)31(41)33(36(24,25)9)44-22(5)38/h14,19,21,24,26-27,31-33,41-43H,3,10-13,15-18H2,1-2,4-9H3,(H,37,39)/t21-,24-,26-,27+,31-,32+,33+,35+,36-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250751

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CNC(=O)OC(C)(C)C)[C@H](OC(=O)CNC(=O)OC(C)(C)C)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C46H72N2O11/c1-25(2)26(3)16-17-27(4)30-19-20-31-29-18-21-33-44(12,13)38(57-35(51)24-48-41(54)59-43(9,10)11)32(56-34(50)23-47-40(53)58-42(6,7)8)22-45(33,14)36(29)37(52)39(46(30,31)15)55-28(5)49/h20,25,27,30,32-33,37-39,52H,3,16-19,21-24H2,1-2,4-15H3,(H,47,53)(H,48,54)/t27-,30-,32-,33+,37-,38+,39+,45+,46-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250752

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-3,11-dih...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CNC(=O)OC(C)(C)C)[C@H](O)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C39H61NO8/c1-21(2)22(3)13-14-23(4)26-16-17-27-25-15-18-29-37(9,10)33(44)28(47-30(42)20-40-35(45)48-36(6,7)8)19-38(29,11)31(25)32(43)34(39(26,27)12)46-24(5)41/h17,21,23,26,28-29,32-34,43-44H,3,13-16,18-20H2,1-2,4-12H3,(H,40,45)/t23-,26-,28-,29+,32-,33+,34+,38+,39-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50250753

((2R,3R,5R,10S,11R,12R,13R,17R)-12-acetoxy-11-hydro...)Show SMILES CC(C)C(=C)CC[C@@H](C)[C@H]1CC=C2C3=C([C@@H](O)[C@H](OC(C)=O)[C@]12C)[C@@]1(C)C[C@@H](OC(=O)CNC(=O)OCC2c4ccccc4-c4ccccc24)[C@H](OC(=O)CNC(=O)OCC2c4ccccc4-c4ccccc24)C(C)(C)[C@@H]1CC3 |r,c:13,t:11| Show InChI InChI=1S/C66H76N2O11/c1-37(2)38(3)26-27-39(4)52-29-30-53-49-28-31-55-64(6,7)60(79-57(71)34-68-63(74)76-36-51-47-24-16-12-20-43(47)44-21-13-17-25-48(44)51)54(32-65(55,8)58(49)59(72)61(66(52,53)9)77-40(5)69)78-56(70)33-67-62(73)75-35-50-45-22-14-10-18-41(45)42-19-11-15-23-46(42)50/h10-25,30,37,39,50-52,54-55,59-61,72H,3,26-29,31-36H2,1-2,4-9H3,(H,67,73)(H,68,74)/t39-,52-,54-,55+,59-,60+,61+,65+,66-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli ECOR1 |
J Nat Prod 66: 1338-44 (2003)
Article DOI: 10.1021/np030211s
BindingDB Entry DOI: 10.7270/Q2GQ6XHC |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM22984

((8S,10S)-10-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-me...)Show SMILES COc1cccc2C(=O)c3c(O)c4C[C@](O)(C[C@H](O[C@H]5C[C@H](N)[C@H](O)[C@H](C)O5)c4c(O)c3C(=O)c12)C(=O)CO |r| Show InChI InChI=1S/C27H29NO11/c1-10-22(31)13(28)6-17(38-10)39-15-8-27(36,16(30)9-29)7-12-19(15)26(35)21-20(24(12)33)23(32)11-4-3-5-14(37-2)18(11)25(21)34/h3-5,10,13,15,17,22,29,31,33,35-36H,6-9,28H2,1-2H3/t10-,13-,15-,17-,22+,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Eco R1 assessed as undigested supercoiled pBR322 DNA concentration |
J Nat Prod 64: 204-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W37W21 |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50242374

(CHEMBL505529 | topostatin)Show SMILES CCCCCCCC(C)CC=C(C)C=CC(=O)C(C)CCC1OC(=O)[C@H](CC(OS(O)(=O)=O)C(N)=O)NC(=O)[C@@H](C)CNC(=O)C(=C)NC(=O)C1C |r,w:14.14,10.9| Show InChI InChI=1S/C36H58N4O11S/c1-8-9-10-11-12-13-22(2)14-15-23(3)16-18-29(41)24(4)17-19-30-26(6)34(44)39-27(7)35(45)38-21-25(5)33(43)40-28(36(46)50-30)20-31(32(37)42)51-52(47,48)49/h15-16,18,22,24-26,28,30-31H,7-14,17,19-21H2,1-6H3,(H2,37,42)(H,38,45)(H,39,44)(H,40,43)(H,47,48,49)/t22?,24?,25-,26?,28-,30?,31?/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >2.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Eco R1 assessed as undigested supercoiled pBR322 DNA concentration |
J Nat Prod 64: 204-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W37W21 |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50008923

((S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-...)Show SMILES CC[C@@]1(O)C(=O)OCc2c1cc1-c3nc4ccccc4cc3Cn1c2=O |r| Show InChI InChI=1S/C20H16N2O4/c1-2-20(25)14-8-16-17-12(7-11-5-3-4-6-15(11)21-17)9-22(16)18(23)13(14)10-26-19(20)24/h3-8,25H,2,9-10H2,1H3/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Eco R1 assessed as undigested supercoiled pBR322 DNA concentration |
J Nat Prod 64: 204-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W37W21 |
More data for this
Ligand-Target Pair | |
Type II restriction enzyme EcoRI
(Escherichia coli) | BDBM50164888
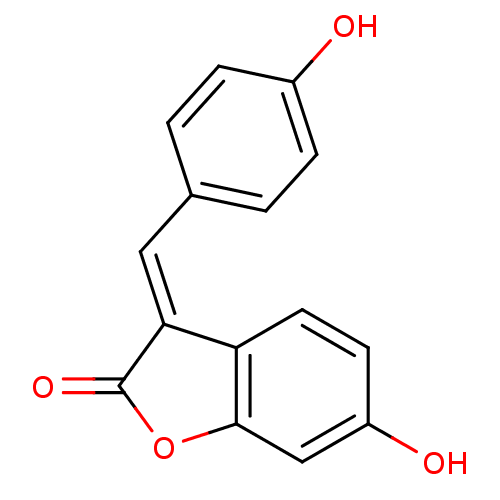
(6-Hydroxy-3-[1-(4-hydroxy-phenyl)-meth-(E)-ylidene...)Show InChI InChI=1S/C15H10O4/c16-10-3-1-9(2-4-10)7-13-12-6-5-11(17)8-14(12)19-15(13)18/h1-8,16-17H/b13-7+ | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kumamoto University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli Eco R1 assessed as undigested supercoiled pBR322 DNA concentration |
J Nat Prod 64: 204-7 (2001)
BindingDB Entry DOI: 10.7270/Q2W37W21 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data